The determination of bulk density and porosity of monolithic materials using a water intrusion method known as the Archimedes method. We follow Method 1 of DIN EN 623-2, which is only suitable for apparent porosity measurements greater than 1%. This is also Method A of ASTM D792. ISO 1183 has essentially the same method.
The method requires the following steps:
- Drying the test sample in an oven.
- Degassing the test sample under reduced pressure.
- Immersing the test piece in water and determining the mass of the soaked test piece.
Please note that this method is not suitable for materials that have an average pore size greater than 200 µm. Also note that because many polymers have a specific gravity less than 1, we have modified this technique to use isopropyl alcohol instead of water.
Sample Preparation
Typically, three test samples are removed from the supplied material with a drill press using 20 mm diamond abrasive coring bits. Approximately 1 gm of test sample is used for the analysis.
Apparatus
Drying oven: An oven which can maintain a temperature of 110 °C ± 5 °C is utilized for drying the test samples.
Balance: An analytical balance equipped with a density measurement apparatus is used to determine the mass of the immersed test sample and the mass of the soaked test piece (Figure 1). The density measurement kit consists of a support plate, pan, bracket, holder for non-floating solids, holder for floating solids, sinker (immersion body), and precision thermometer with holder.

Evacuating equipment: A vacuum pump capable of reducing the pressure to a value of 0.040 Pa (0.0040 mbar) is used for the degassing the test pieces.
Immersion liquid: Distilled water is used as the immersion liquid. When poorly wetting liquids such as water is used, it is possible that air bubbles will adhere to the submerged solid test piece and sinker. Due to their buoyancy, air bubbles could affect the results. To improve the accuracy of test results, a couple of drops of wetting agent are added to the distilled water before submerging the test pieces. The change of density caused by the addition of the wetting agent to the water is negligible.
Procedure
Determination of the mass of the dry test samples: The test samples are kept in an oven at 110°C over night to ensure that test samples are completely dry. The test pieces are then placed in the desiccator until they have cooled to room temperature. They are removed for the desiccator and weighed immediately. The mass thus determined is the mass of the dry samples (m1).
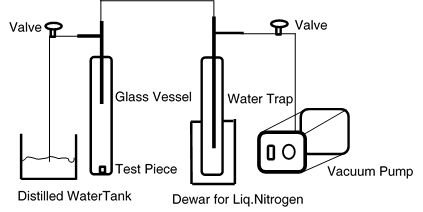
Soaking of the test samples: The test sample is placed in the glass vessel which is evacuated to a pressure of less than 2500 Pa (Figure 2). The vacuum is maintained at least for 15-20 minutes. In order to ensure that all the air trapped in pores has been removed from the test sample, the vessel is isolated from the vacuum pump by closing the valve. A manometer monitoring the pressure checks that the pressure does not rise due to further air coming out of the test sample. Subsequently distilled water is introduced in to vessel in a such way that the test sample is completely submerged in distilled water. Then, the vessel is maintained under reduced pressure for 30-40 min by opening the valve that is connected to the vacuum pump. Then the valve to the pump is shut and the vessel is opened to ambient air pressure for another 30-40 mins to ensure that water completely penetrates the test sample.
Determination of the apparent mass of the immersed test sample: The test sample is suspended in the distilled water and weighed while completely immersed in liquid (Figure 3). The mass thus determined is the mass of the immersed test piece (m2).
Determination of the apparent mass of the soaked test sample in air: The test sample is removed from the liquid and immediately wiped with a tissue to remove the droplets and the surface film of the liquid while avoiding drawing liquid from the pores by performing the wiping operation very quickly. The test sample is then weighed immediately (Figure 4). The mass thus determined is the mass of the soaked test sample (m3).

Calculating Density and Porosity Values

It is a rather neat result that three such weight measurements can yield so much information about the solid material lattice density, the average density of the sample material, and the percentage of the porosity volume.
ASTM D792-20 — Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement