|  |
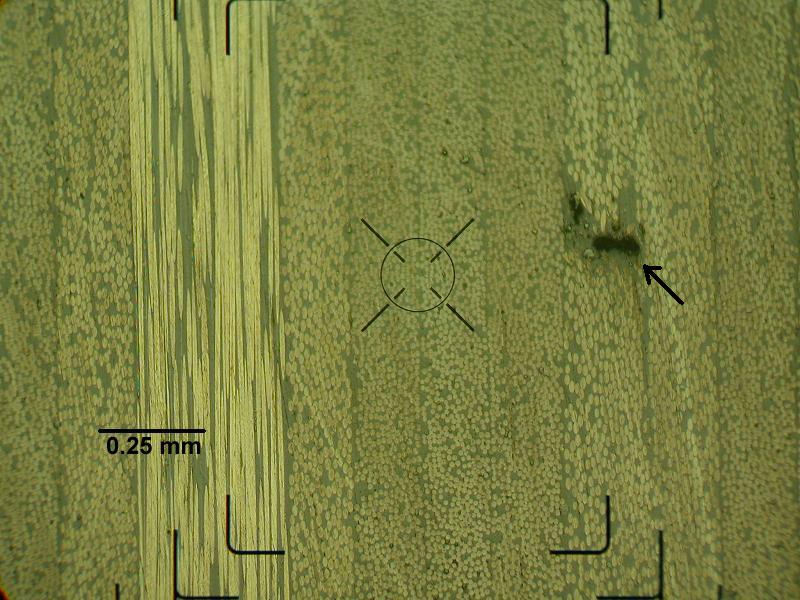
Two samples of mortar for a renovation project of a historic structure. The client was trying to find mortar that matched the original structures.
Many of the materials we analyze are composite materials. Composite materials analysis often requires the use of multiple analytical techniques to properly characterize the materials and the interfaces between them which often contribute in important ways to the properties of the materials.
Composite materials are most commonly polymer resins with fibers, whiskers, or particles embedded in the resin. The fiberglass epoxy composite material comes most frequently to mind, but in actuality many, many engineering materials are composite materials. In addition to the polymer epoxy resin, polyimide and polysulfone resins are in widespread use. Polyester, vinyl ester and polyurethane resins are also in use. In addition to E-glass fibers, the most common reinforcing fiberglass material, S-glass, carbon (pan or pitch) fibers, aramid (Kevlar-49), aluminum oxide, and silicon carbide fibers are in use. Whisker materials include graphite, silicon carbide, aluminum oxide, and silicon nitride. Metal wires may also be embedded, such as steel, tungsten, or molybdenum.
In reality, composite materials analysis is performed on many other resins and embedded materials. Carbon black in rubber makes that a composite material. Titanium dioxide, zinc oxide, and calcium carbonate in paints make them composite materials. Calcium carbonate in polyvinylchloride (PVC) pipe makes it a composite material. A clearcoat layer loaded with fine silica gel particles is also. A tabletop material of polyethylene or epoxy may be loaded with aluminum trihydrate (a fire retardant), silica, or aluminum oxide particles. A metal primer used to provide corrosion resistance is filled with metal oxide particles in many cases and so is a composite material. Many smart materials are composite materials. Adhesives and sealants are often filled with particles such as silica, alumina, calcium carbonate, zinc oxide, carbon black, bentonite clay mineral, and fire retardant particles such as antimony oxide. Many plastics are filled with these same fillers or with metal particles to provide radiation shielding. Concrete and mortars are other composite materials. Wood and bone are examples of inherent composite materials. Wood chips in polyurethane resin is another composite material. We have performed analyses of all of these composite materials at AME.
In all such cases, the chemistry at the interfaces of the additives with the polymer resin is of great importance for many material properties. Does the resin properly wet the reinforcing additive material surfaces? Do the fibers suffer pull-out upon mechanical failure? Where does failure actually occur when the additives are ripped from the resin along a fracture interface? The percentage weight of the fiber, whisker, and particle additives is an important property. The orientation of fibers and whiskers is important. Do particles agglomerate or migrate to the surface of the resin material over time? The resin thermal properties are critical, as is its resistance to chemical attack, thermal degradation, and UV degradation. Composite materials analysis can be quite complex and a materials analysis laboratory undertaking such projects needs very intelligent and innovative scientists and the correct materials analysis tools to characterize and crack many of the problems of composite materials.

Cross Section Microscopy Analysis with Inspection Microscope, Metallographic Microscope, Keyence 3D Digital Microscope, or SEM:
- Void and crack detection
- Check ply orientation
- Measure layer thickness
- Determination of failure mode
- Detect voids and measure void density and sizes
- Determine if fill particles are agglomerating or migrating to interfaces
Thermogravimetric Analysis or TGA:
- Measure weight of fill materials such as fiberglass, carbon black, silicon nitride, carbon fibers, silicon carbide, calcium carbonate, aluminum trihydrate, magnesium, hydroxide, silica, talc, mica, and clay particles and whiskers
- Measure the respective weight of each polymer of a copolymer material
- Void content by volume after determining the initial volume and weight using our Mettler Toledo XS105 balance with volume/density apparatus. Then the resin weight and the final fiberglass or particle weight are determined using our ISI 1000 TGA.
- Measure the water content absorbed
- VOC emissions or outgassing upon further cure or heating
- Determine the temperature at which water is released by hydrated fill particles
Thermomechanical Analysis or TMA:
- Thermal expansion or CTE properties
- Determine the degree of polymer crystallinity
- Measure softening temperatures and glass transition temperatures
- Swelling characteristics in various solvents or liquids
- Measure shrinkage due to the volatilization of adsorbed liquids such as water, solvents, fuels, or oils
Differential Scanning Calorimetry or DSC:
- Measure the glass transition temperature
- Determine the degree of cure or energy of cure
- Endothermal melting or phase changes detected and measured, which can be used to quantify the melting phase or may indicate impurities and isomers
- Measure the endothermic energy of water release
- Measure the oxidation temperature and energy of reaction of components such as the matrix polymer or binder
- Measure exothermal energy release due to internal reactions
FTIR or Infrared Spectroscopy Analysis:
- Determine organic resin identity
- Detect organic layers on fiberglass or fill materials
- Detect surface coatings
- Detect carbonates, phosphates, nitrates, nitrites, and water
GC-MS or Gas Chromatography – Mass Spectroscopy Analysis:
- Determine what organic chemicals which are either volatile or can be dissolved in a solvent are in the resin.
- Determine what organic chemicals may be on the surfaces of fibers or fill particles to be added to a composite material.
- Detect chemicals that may have infiltrated a composite material in use.
- Determine how a resin might have degraded in use or due to improper manufacturing processes.
WD-XRF or Wavelength Dispersive X-ray Fluorescence Spectrometry
- Quick determination of composition of inorganic fill materials
- Can measure relative nitrogen to carbon concentrations in resin components of sheet materials or composite coatings on substrates
- May be used to measure paint or other coating thickness on a substrate once that thickness has been measured by cross section for the same coating material at several thicknesses, speeding up further thickness measurements
- Polymer matrix composition with quantitative elemental and functional group chemical analysis
- Contamination on surface affecting adhesive bonding such as from mold release agents or short chain length airborne silicones
- Surface chemistry of fiberglass and fill particle materials affecting bonding or water release properties
- Identification and thickness measurement of silane coupling agents on additive surfaces
- Residue measurements and detection on extruded fibers of such compounds as calcium or zinc stearate
- Detection of sizing or sizing residues on fibers
- Identification of solid residue due to fill materials after TGA burn-off of polymer matrix
- Identification of E or S fiberglass after TGA burn-off of polymer matrix
- Surface chemistry of fibers, whiskers, or particles due to surface treatments such as cleaning, abrasion, plasma treating, environmental exposure, or degradation
- Interphase chemistry at interfaces where local compositions vary over 10 nm and even 1 micrometer scales in various cases compared to the bulk chemistry further away from the interface
- Identify the chemical phases of crystalline fill particles
- Determine the degree of semi-crystalline structure of a polymer resin
Profilometry or Surface Roughness Measurements
- Tensile strength, elasticity, stress-strain curves, on-set of plastic deformation
- Compressive strength
- 3-point and 4-point bending characteristics
ASTM Test Methods:
- ASTM D2584 – Standard Test Method for Ignition Loss of Cured Reinforced Plastics
- ASTM D2734 – Standard Test Method for Void Content of Reinforced Plastics
- ASTM E831 – Standard Test Method for Linear Thermal Expansion of Solid Materials by Thermomechanical Analysis
- ASTM E1131 – Standard Test Method for Compositional Analysis by Thermogravimetry