Introduction:
This study explores polymer break down through FTIR analysis, XPS, and residual gas mass spectroscopy. It focuses on a composite aerospace structure tested for flame resistance. We aim to understand how the material behaves under extreme conditions.
Analysis of Polymer Degradation Products
During the flame resistance test, we found melted red material near metal hardware. We also observed black residue and a thin, milky clear layer. We used XPS and FTIR analyses to test these materials. The results required further investigation.
FTIR Spectra Analysis of Polymer Degradation
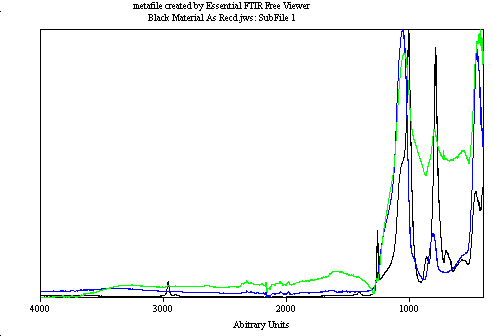
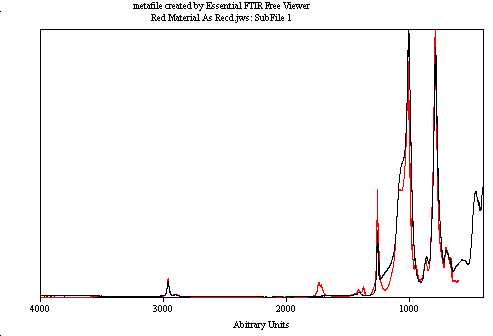
We analyzed the FTIR spectra of the broken down products—melted red (black spectrum), charred black (green spectrum), and milky clear material (blue spectrum). These spectra revealed similarities with known materials. The red material matched Permatex RTV 28BR. We observed slight methyl group loss and oxidation. XPS confirmed a 2.5% decrease in carbon and a 21% increase in oxygen as hydroxyl groups. These changes are significant and indicate chemical alterations.
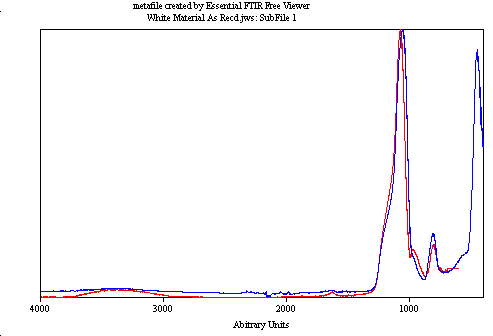
We found that the milky clear layer matched a silicon gel material in FTIR databases. XPS identified it as highly disordered silica, lacking significant hydroxyl groups. This was an interesting find that needed further exploration.
Experimental Validation
We did laboratory overheating of Permatex RTV 26BHTR, a red gasket material. This process copied similar break-down products. The products included melted red, charred black, and clear silica like material, based on heating conditions. This suggests overheating of siloxane materials, potentially under-cured or contaminated by flammable volatiles. These results were consistent with our initial findings.
Conclusion and Recommendations
Our study shows that siloxane materials are vulnerable to thermal break down, releasing combustible short-chain siloxanes. Proper curing and contamination control are crucial to prevent unexpected flammability in composite structures. Ensuring these precautions can help avoid future issues.
Contact Us:
For inquiries regarding FTIR analysis and polymer concerns, please visit our “Contact Us” page. You can also email Dr. Griffin Spence at Griffin@andersonmaterials.com, or Dr. Priyanshu Banerjee at Priyanshu@andersonmaterials.com, or call 1-410-740-8562. We are here to help and answer any questions you may have.