Elemental and Crystalline Phase Analysis for Mineral Composition by XRF and XRD

Wavelength-Dispersive XRF Analysis
We perform XRF elemental analysis first to guide us in identifying which of the many tens of thousands of crystalline chemical phases may be present in a potentially very complex XRD spectrum. After grinding the sample to a fine powder, we place it in a plastic cup with a thin polypropylene film covering the 29 mm aperture in the bottom of the cassette into which we insert the plastic cup. We analyze a solid sample capable of supporting itself over the 29 mm aperture in vacuum, but we analyze a powder sample in helium. Our Thermo Scientific ARL PERFORM’X 1500 sequential XRF spectrometer detects and measures elements from sodium through uranium under a helium atmosphere. The results in weight percent are given below.
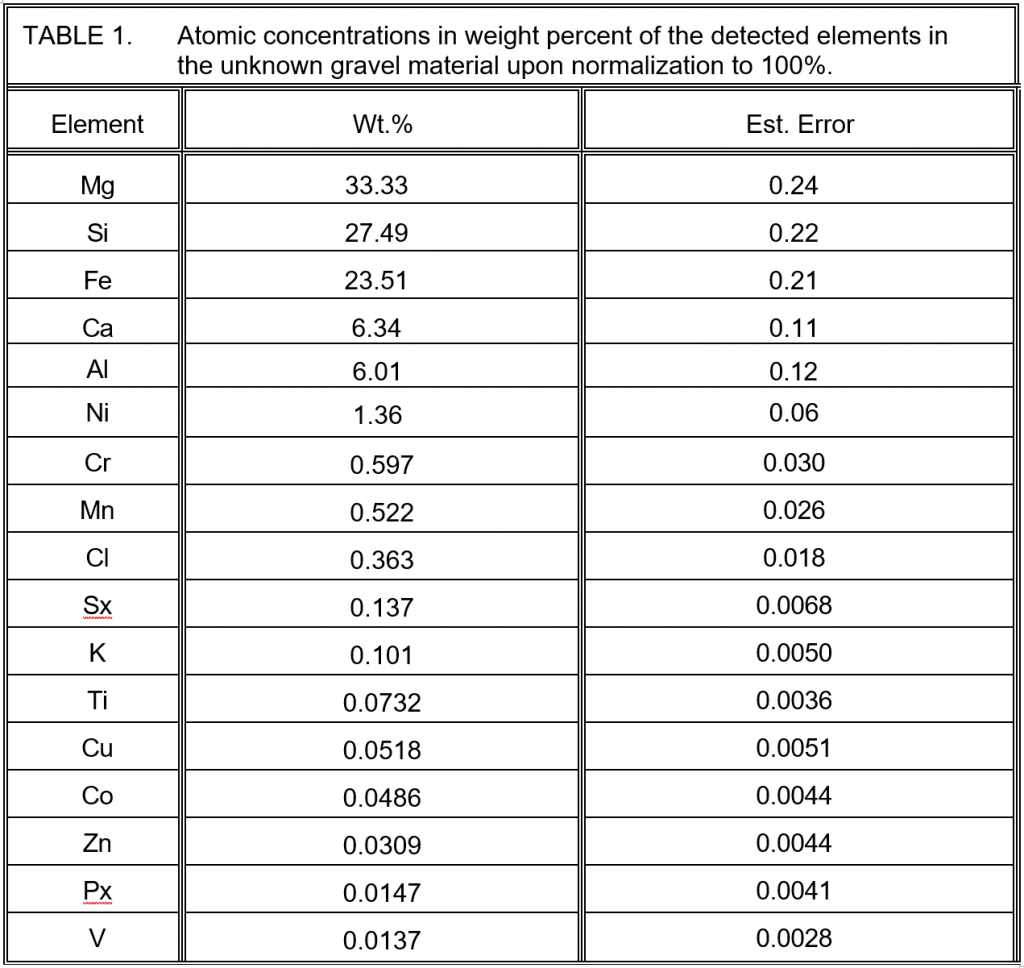
Sx is sulfur as sulfate and Px is phosphorus as phosphate.
XRD Analysis to Identify Crystalline Chemical Phases
We prepared a finely ground sample from the unknown mineral for analysis in our Rigaku MiniFlex 6G XRD instrument.
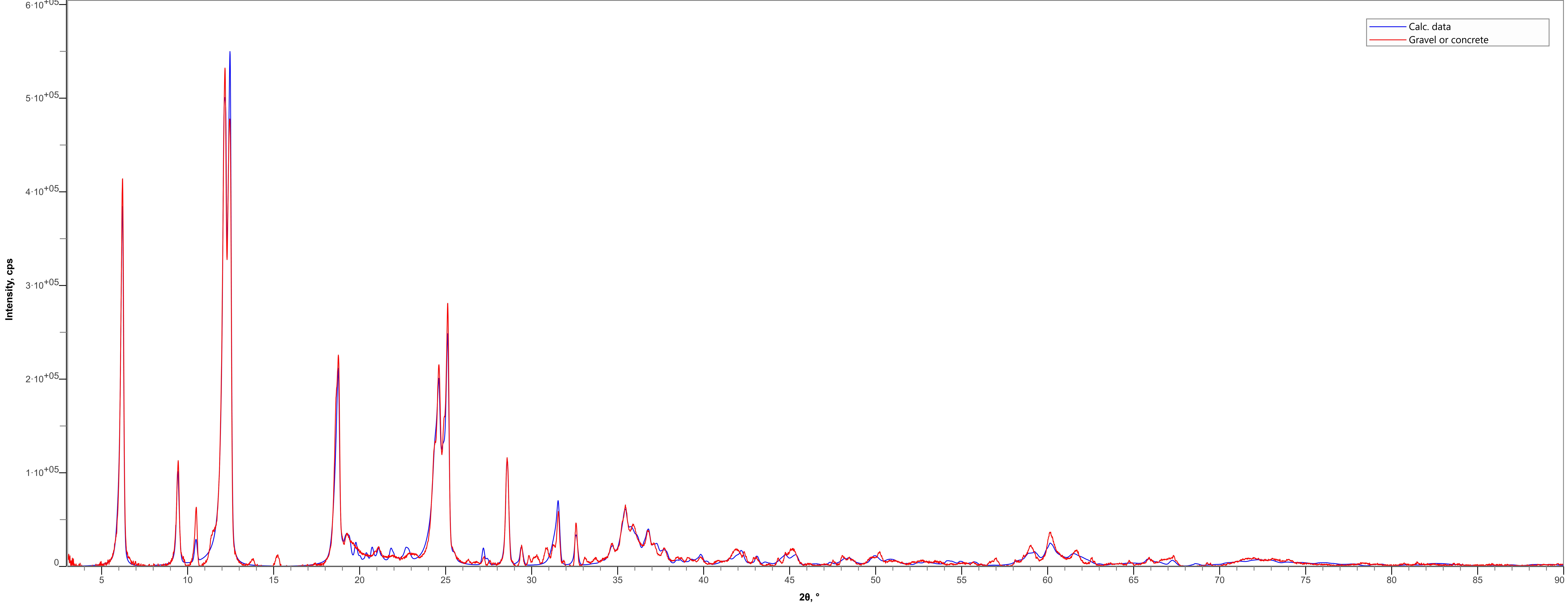
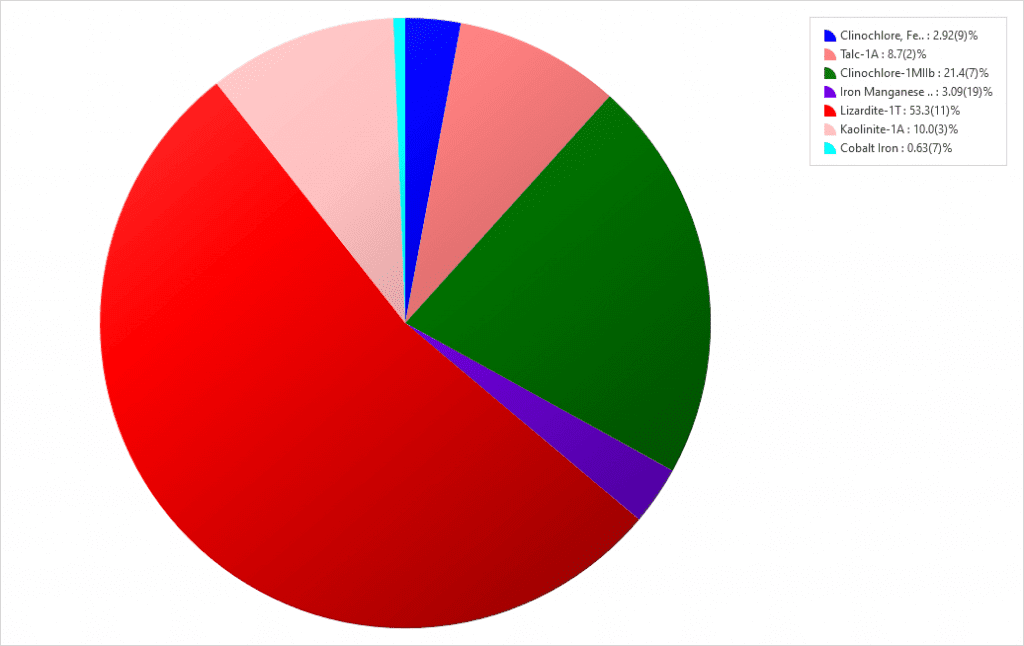
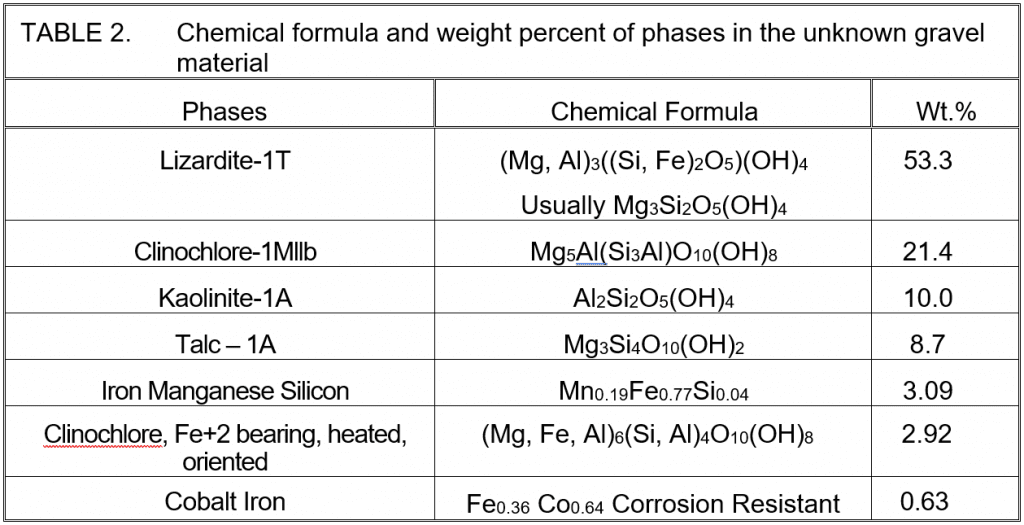
Lizardite, a mineral in the serpentine subgroup, is the predominant phase in the gravel material. Clinochlore and Kaolinite are clay minerals of the phyllosilicate group of silicate minerals. The iron manganese silicon and the cobalt iron phases that fit the data are questionable because they are not oxidized. The concentration of manganese is fairly consistent with the XRF result. While XRF detects cobalt, the amount is too low to support the quantity implied by the XRD fit. Therefore, consider the cobalt iron improbable and the iron manganese silicon very questionable. Between them, they leave 3.72 weight percent of the composition unknown.
Contact Us
If you have any questions or need our assistance, please feel free to contact us. Our team of scientists is happy to work with your business to figure out any unique issues with your materials.