We used XPS analysis to detect silicone contamination on two 3M film tapes. Our objective was to assess whether these tapes, with acrylic adhesive on polyimide backing, are acceptable for applications where surfaces must be free of silicones and silicon-based chemicals.
Analysis of 3M Film Tapes
The first tape we tested is called Polyimide Film Electrical Tape 1205. It has a thermosetting acrylic pressure-sensitive adhesive. According to the 3M Data Sheet dated February 2017, it can be used at temperatures up to 155°C.
The second tape is called 3M Low Static Polyimide Film Tape 7419. According to the Technical Data Sheet dated August 2014, this tape also has an acrylic adhesive. It is used for PCB solder masking and other high-temperature applications. The data sheet claims the tape stays in place during processing and removes cleanly from electronic substrates, even after 10 minutes at 260°C. It also states that this tape has a non-silicone adhesive formulation to reduce silicone contamination.
Silicone Detection Results: 3M 1205 Film Tape
Elemental XPS Spectrum of 3M 1205 Film Tape Adhesive Surface
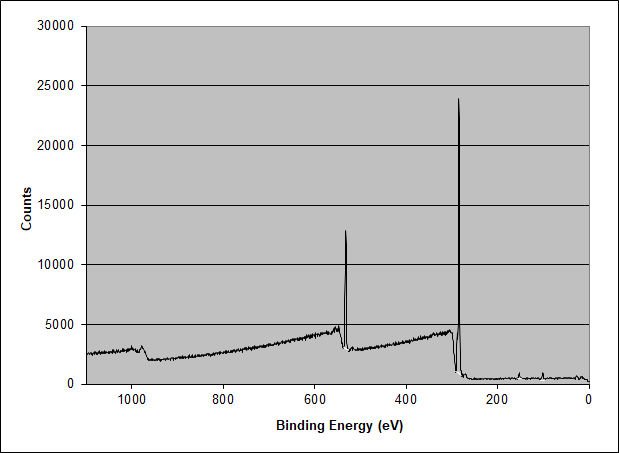
The elemental XPS spectrum shows peaks for Si 2p, Si 2s, C 1s, O 1s, and O Auger (about 975 eV). The surface’s elemental composition in atomic percent is as follows: Carbon 82.21 at.%, Oxygen 15.49 at.%, and Silicon 2.30 at.%. The acrylic adhesive should only contain carbon and oxygen, so the presence of silicon is unexpected. What is the chemistry of the silicon?
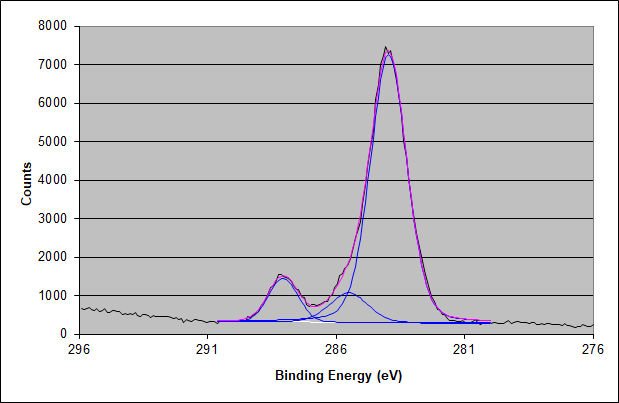
Carbon 1s Spectrum of the Surface
The carbon 1s high-energy resolution XPS spectrum shows two higher binding energy peaks of nearly the same area expected for an acrylic adhesive. The higher binding energy peak has slightly more area due to some acrylic acid content.
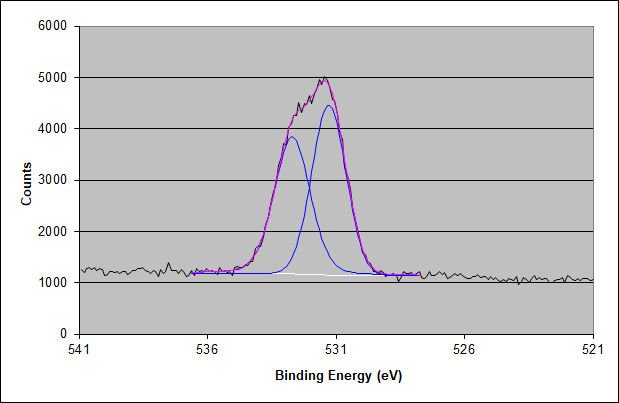
Oxygen 1s XPS Spectrum of the Acrylic Adhesive Surface
The XPS spectrum of the oxygen 1s peak shows the two oxygen atoms expected in the acrylic adhesive molecule.
Silicon 2p High-Energy Resolution XPS Spectrum of the Surface of the Acrylic Adhesive
When we compensated for the 1.05 eV downward shift in binding energy caused by surface charging, the Si 2p binding energy was 102.15 eV. This value corresponds to a very short chain-length dimethyl siloxane. Dimethyl siloxane is the most common type of silicone, and short chain-length dimethyl siloxane is the most common form of airborne silicone contamination. This contamination can decrease surface wetting and degrade adhesive bonding strength at interfaces. The 2.3 at.% silicon in the form of PDMS is slightly less than the 3 at.% usually found at the interface of failed adhesive bonds. Ultimately, the 3M 1205 film tape proved entirely unsuitable for use as a contact silicone transfer test material. We couldn’t use it to determine whether a surface in a customer’s facility was contaminated with silicone.
Silicone Detection Results: 3M 7419 Film Tape
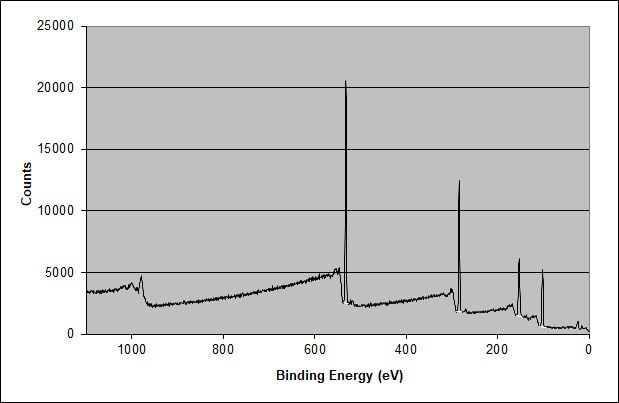
XPS Elemental Survey Spectrum of the Surface of the 3M 7419 Film Tape
Unfortunately, the Si 2p and Si 2s peaks are prominent in the surface of this non-silicone acrylic adhesive formulation. The atomic percentage composition is 42.99 at.% carbon, 29.89 at.% oxygen, and 27.12 at.% silicon in the outer 10 nm of the adhesive surface. This is close to the expected 2:1:1 ratio of carbon:oxygen for a poly(dimethyl siloxane). This finding shows that the outer 10 nm of the surface is entirely made of poly(dimethyl siloxane) (PDMS). The high-energy resolution XPS spectra of the carbon 1s, oxygen 1s, and silicon 2p peaks confirm this and also explain why the carbon ratio is slightly depressed and the oxygen ratio is slightly enhanced compared to the expected ratios for poly(dimethyl siloxane).
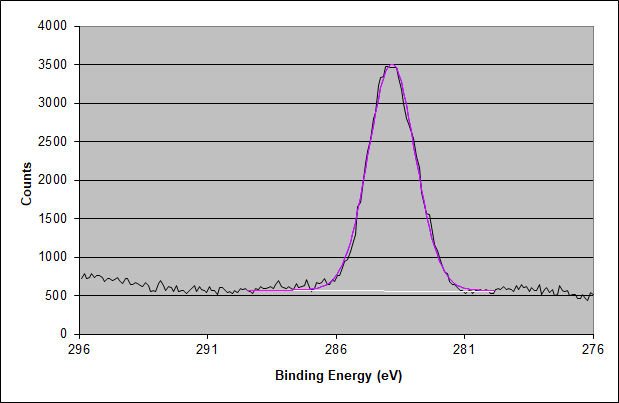
High-Energy Resolution Carbon 1s XPS Spectrum of the 3M 7419 Adhesive Surface
The two higher binding energy components found in an acrylic adhesive are entirely missing. Thus, no acrylic adhesive is present in the outer 10 nm of this adhesive surface.
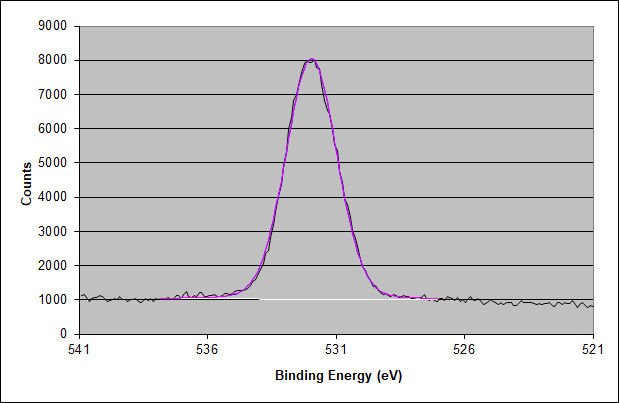
High-Energy Resolution XPS Spectrum of the Oxygen 1s Photoelectron Peak of the Adhesive Surface
Similarly, the double oxygen peak characteristic of acrylic adhesive is absent.
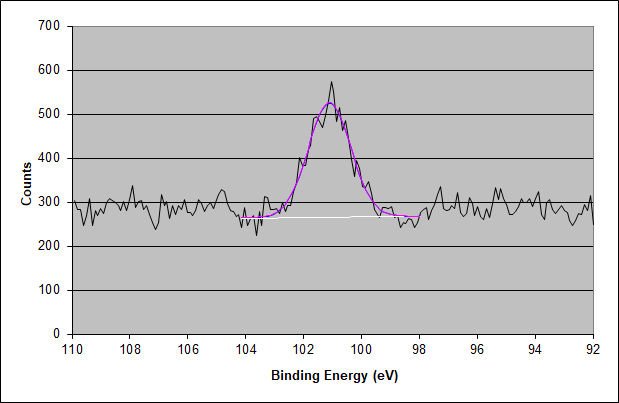
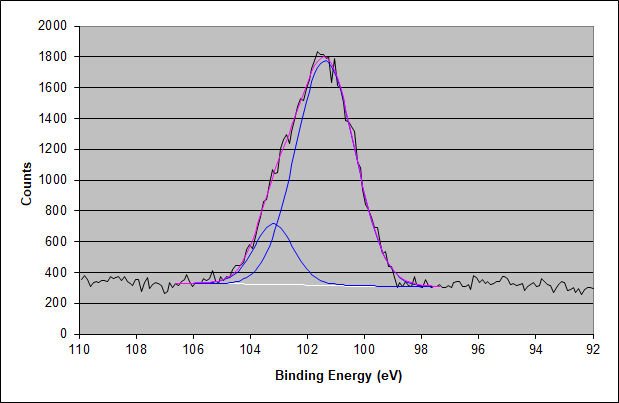
XPS High-Energy Resolution Silicon 2p Photoelectron Peak for the Surface of the Adhesive
After correcting for the 1.17 eV charging shift, the lower binding energy and the main Si 2p peak have a binding energy of 102.55 eV. This value is characteristic of a mid-chain length PDMS, which is commonly found in pressure-sensitive silicone adhesives. The smaller higher binding energy component is likely due to severely heat-degraded PDMS material, which becomes carbon deficient and enriched in oxygen. Alternatively, it may be due to a very fine silica powder. This explains why the elemental ratio is not precisely 2:1:1 for carbon:oxygen as expected for a mid-chain length dimethyl siloxane.
Conclusion
Finding a truly silicon-free and silicone-free adhesive tape proved to be a difficult task. Although we have identified a tape for use in our tape transfer silicone detection and measurement test kits, we continue to search for alternatives.
Contact Us
For more information or to discuss your silicone contamination concerns, please contact us. We are here to assist with inquiries and provide personal solutions for your engineering and research needs.