Our client reported a wet clay odor from installed floor tiles when they got wet. We conducted an analytical investigation to understand the cause. Using optical microscopy, XPS surface analysis, and XRF sub-surface analysis, we identified the issue.

Analysis and Findings
The ceramic floor tile had a total thickness of 9 mm, with a 0.5 mm hard, white layer over a baked clay substrate. A surface glaze was applied on top of this layer.
Observations
- Distilled water placed on the tile surface beaded into puddles and left water spots after drying.
- The deposits left on the glaze indicated that water was leaching ions from the underlying materials, suggesting defects in the glaze.
Visual Evidence
- Optical Microscopy: Showed round defects in the glaze, indicating bubbles that might provide pathways to the underlying material.

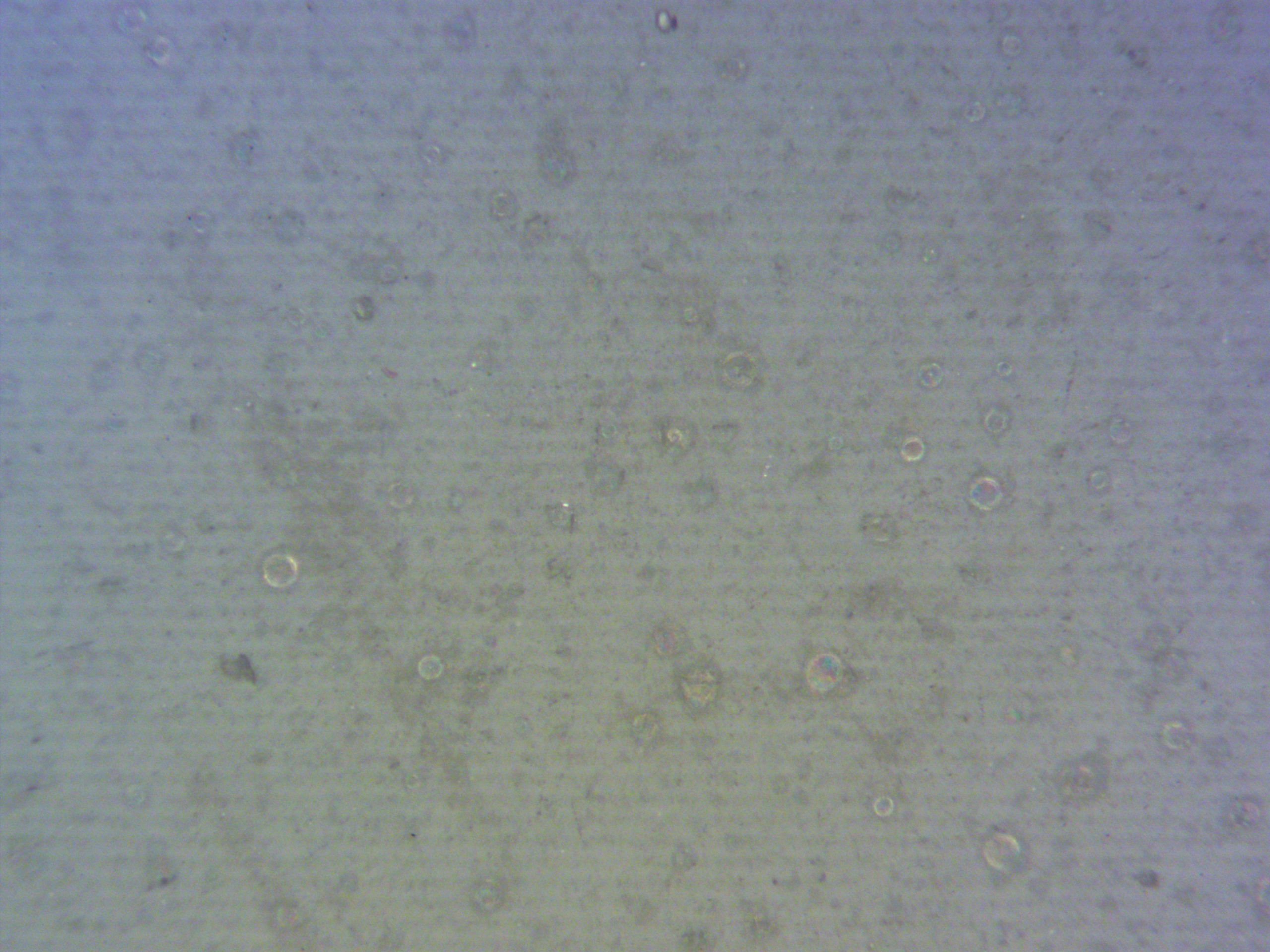
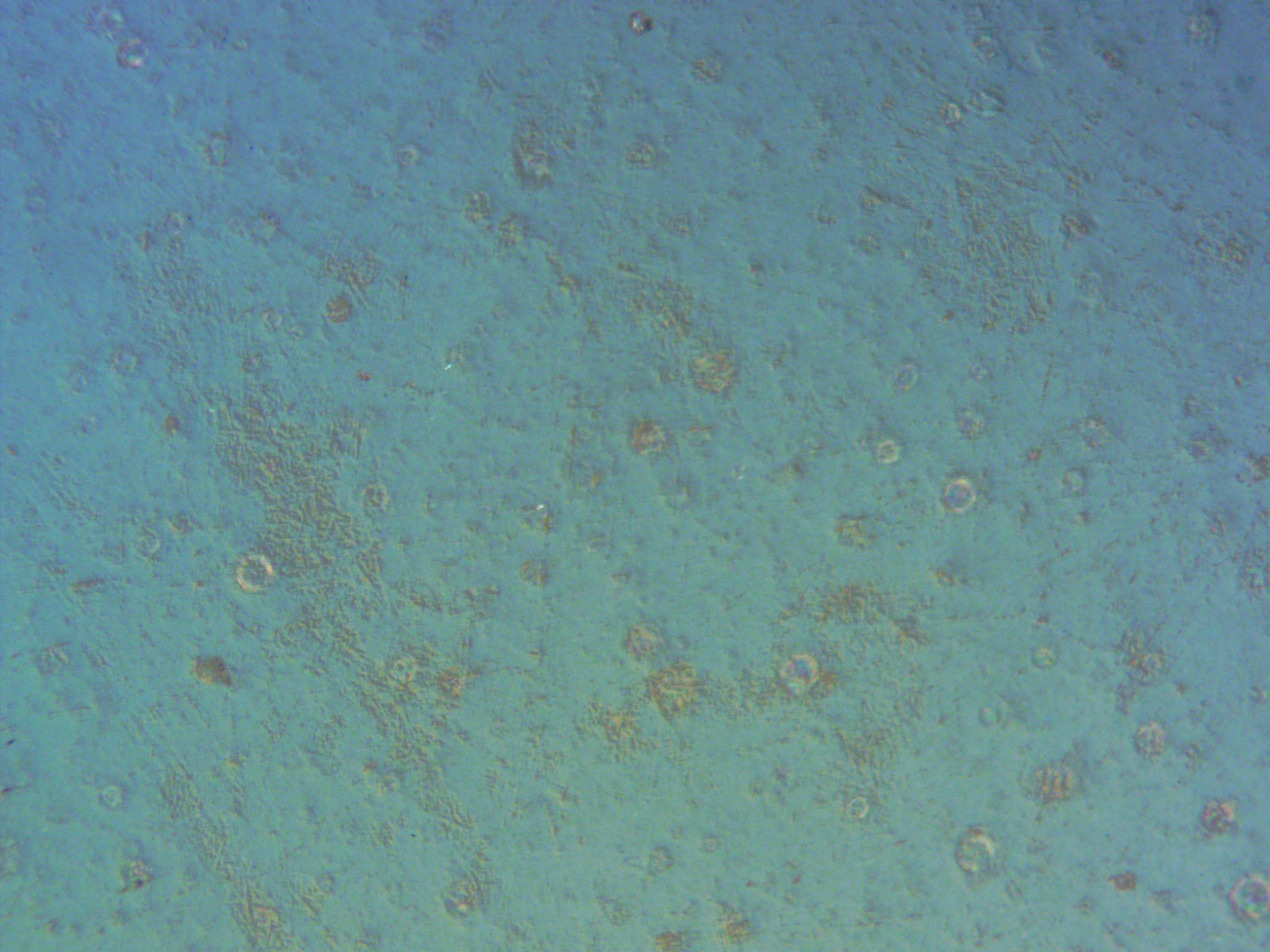
Chemical Analysis
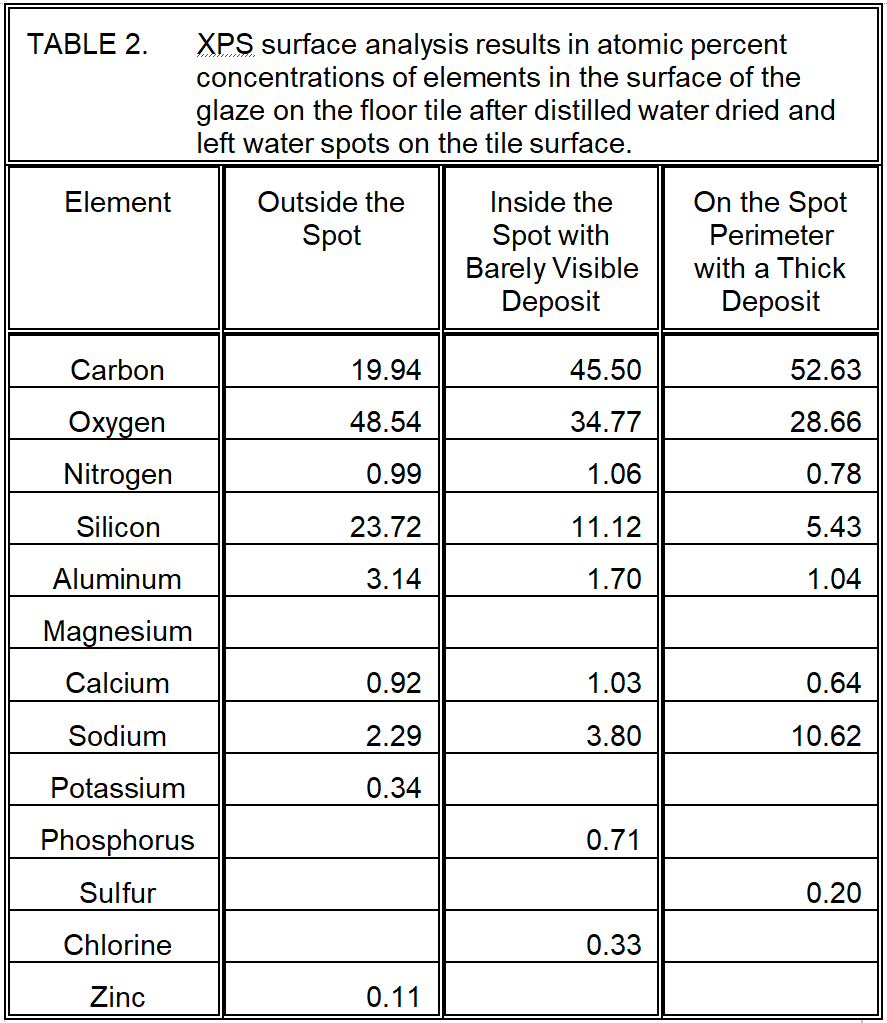
- XPS Surface Analysis: Conducted outside a water spot, inside a water spot, and at the heavy deposit at the water spot perimeter. The analysis showed increased organic and carbonate carbon concentrations in the water spot deposits compared to the clean glaze surface.
- XRF Analysis: Used to determine the composition of the hard, white layer under the glaze, revealing a significant concentration of sodium, which indicated a source of the leached ions.
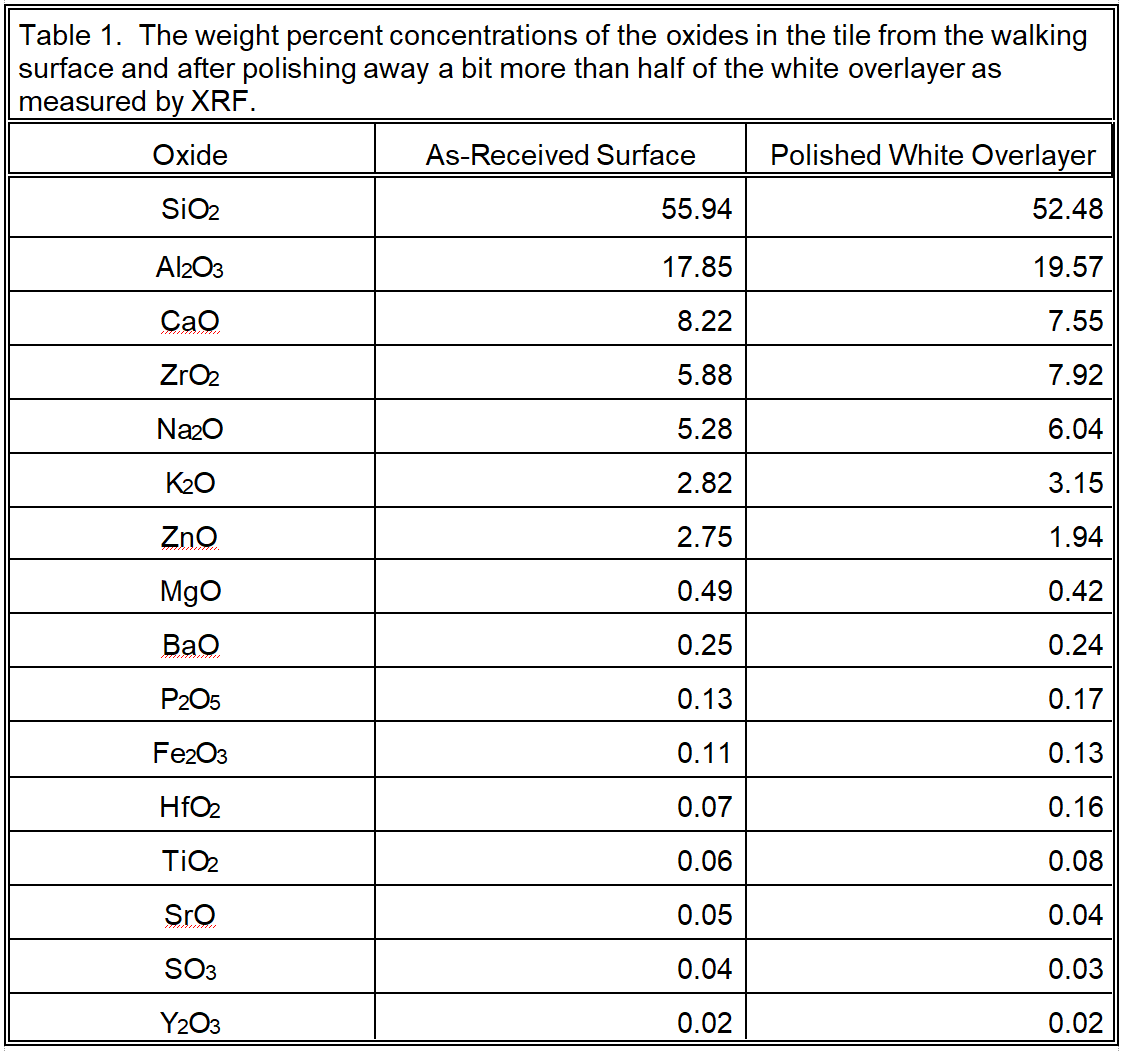
Conclusion
- The distilled water left deposits of organic material and sodium carbonates on the tile surface.
- The surface glaze should have prevented the dissolution of its components, but the observed microscopic bubbles created pathways to the sodium-rich underlying layer.
- This layer, not designed to prevent leaching, led to undesirable water spots and the wet clay odor reported by the client.
The complete analysis report is available for review.
For any inquiries or further information, please contact us.