Evaluating Intergranular Corrosion with Metallography and XPS Chemical Analysis: Corrosion Case Study #1
In this electro case study, a Ni-based alloy material of a reactor flange exhibited various forms of cracking in a high-temperature chemical processing environment. The polished cross section at the top shows a surface that has suffered general corrosion. In addition, apparent cracking with varying degrees of severity had penetrated the surface uniformly across the entire specimen surface.
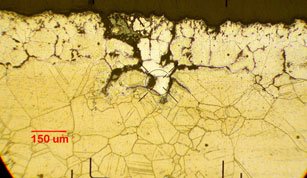
The polished cross section was then subjected to an electrolytic etch. Its microstructure is shown in the image on the bottom. Intergranular corrosion (IGC), the primary form of attack, was found to be largely confined to the region of smaller grains found close to the surface. To a depth of roughly 50 μm the grain boundaries were all but obliterated and the coloration of the material varied from the bulk due to the formation of a mixed corrosion product layer at the specimen surface. At 50-100 μm the color gradually darkened to match that of the bulk metal, and the well-defined cracks were confined primarily to grain boundaries.
A bright group of grains formed a wedge shape that extended roughly 300 μm below the surface of the specimen, as clearly seen after etching. The intergranular attack surrounding this wedge was generally more extensive than in the surrounding material. The change in coloration suggests a change in phase between the wedge and the surrounding bulk material. The deeper and wider grain separation suggests an enhanced corrosion cracking mechanism, perhaps due to galvanic coupling between the dissimilar metallic phases.
X-ray Photoelectron Spectroscopy (XPS) Evaluation
A powdery yellow reaction product was found on the surface of the Ni-alloy specimen shown in the figure above. A sample of this powdery material was collected and analyzed by XPS, revealing the composition shown below in atomic percentages. Sources of the detected elements were determined to be from the material itself, neighboring materials in the working environment, and the environmental conditions created by the process.
| Element | Yellow Reaction Product Composition (at.%) | Source |
|---|---|---|
| Al | 1.65 | Aluminosilicate Materials |
| Si | 3.34 | Aluminosilicate Materials |
| S | 0.56 | MoS2 Lubricant |
| Cl | 0.70 | Processing Environment |
| C | 69.49 | Processing Environment / Alloy Surface Segregation |
| Ca | 0.64 | Processing Environment |
| N | 1.65 | Processing Environment |
| O | 21.16 | Processing Environment |
| Cr | 0.16 | Sensitization / Intergranular Corrosion |
| Na | 0.65 | Processing Environment |
Bad actors such as Cl– and S2- were detected in this case study at the surface of the metal specimen. Both are known to promote general corrosion and a variety of cracking mechanisms in suitable environments. The presence of Cr in the surface corrosion product implies that it was lost from the alloy, thereby reducing its corrosion resistance at the surface. This can be expected if sensitization has occurred in the high temperature operating environment. High concentrations of graphitic C at the surface are expected for C-bearing alloys, especially if high temperatures are involved. The high C concentration seen at the surface implied that similar migration probably occurred at the grain boundaries, which are energetically more similar to surfaces than to bulk material. In another area of the flange, a massive crack propagated through a bolt hole and onward far into the interior. We forced the continuation of this crack further in the laboratory and found the crack tip and the newly exposed area ahead of the crack tip to have high graphitic C concentrations and Cl– concentrations. No sulfur was detected at the deep crack tip.
The presence of damaging anions in combination with the suggestive evidence for Cr and C grain boundary segregation supports the metallographic evidence for IGC.
Conclusions
In combination with data collected from specimens taken from additional areas within the Ni-alloy sample, the following results were determined in the case study:
- The high temperatures to which this material were subjected caused sensitization (chromium carbide formation at grain boundaries), leaving neighboring areas within grains depleted of chromium and consequently more susceptible to corrosion and intergranular cracking.
- Phase variations in the bulk material can lead to galvanic attack between grains further driving the corrosion cracking progress.
- High temperatures also accelerated the migration of graphitic carbon from the alloy to the exterior surface and the grain boundaries, weakening the microstructure mechanically and providing channels for the entrance and penetration of damaging anions to substantial depths into the material.
- The presence of anions such as S2- and Cl– from the processing environment contributed to crack initiation by attacking susceptible areas at the surface.
- Additional XPS data (not shown) of a stress-corrosion cracking (SCC) surface from the same Ni-alloy sample showed that it was the Cl– ions that penetrated the deep cracks to further promote the corrosion cracking mechanism. S2-, while present at the surface as expected from the use of sulfur-based lubricants, was not detected in the SCC crack.
Recommendation
IGC and SCC occur from a necessary combination of factors including alloy composition, environment, and in the case of SCC, tensile stress. To prevent these forms of corrosive attack only one factor need be altered or removed. Since in this application neither the processing environment nor the presence of tensile stresses could be easily addressed, a change in alloy was recommended at the end of the case study. The current alloy was unable to tolerate sulfide and chloride exposure in the required temperature ranges.
Contact us regarding your electrochemistry and corrosion testing needs.