Interface analysis is a major specialty of this laboratory. A surface is a special case of the more general interface, in which an interface is a termination of one material and the start of another. For the surface, air is the usual other material to the termination of a solid or liquid material, though in some of our analyses the other side is vacuum. Between the interior of a material and either air or vacuum, there is often a plane of altered material, such as the oxide on a metal or semiconductor or a hydrated layer on a mineral or inorganic compound. The surface of a material commonly has more contamination than does the bulk of the material. The near surface of a material has often undergone corrosion or other material degradation. The chemistry of a surface may change rapidly with depth from the immediate surface over scales from a nanometer to a few micrometers. A metal alloy, for instance, may have oxides, hydroxides, carbonates, differing concentrations of graphitic and carbide forms of carbon, and differing metallic component compositions at the immediate surface, in the near-surface, and in the bulk of the metal. A stainless steel will commonly have enhanced chromium in the oxide layer and a depletion zone beneath the oxide in which there is less chromium than is found in the bulk alloy.
The grain boundaries of a metal or a metal alloy are another example of an interface. The grain boundary chemistry is also often different than is the bulk grain chemistry. The near surface of the grain is also different than the interior in composition. The grain boundary area is a comparatively easy diffusion pathway. Grain boundaries will often have much more carbon in them, whether a metal alloy or an inorganic polycrystalline material.
In a ceramic, the sintered particles will often have a different surface chemistry than that in the bulk of the particle. When a ceramic material is fractured, the fracture will largely follow the original surfaces of particles, since the fusion of one particle to another will commonly involve only a fraction of the original particle surface area. Analysis of a fractured surface will then clearly reveal some of the altered chemistry resulting from the original particle composition differences at the particle surface. It will also be rich in any sintering agent added to make the sintering of the ceramic particles easier. This combination of altered chemistry from the ceramic particle surfaces and from sintering agents is an interfacial zone in the ceramic. This is an example of interface analysis.
Interfaces are of critical importance in adhesive bonding with respect to the differing chemistry of both the adhesive and the adherend near the interface and due to any contamination that may be at the interface. When an adhesive bond failure occurs, we perform interface analysis to determine the cause of the failure. The surface chemistry and the surface area of a catalyst are critical in its usefulness and in its poisoning. Interfaces are the initiation sites of corrosion and other materials degradation reactions and often control the rate at which further corrosion develops. They affect the way an overlayer of deposited thin film or coating material grows and develops its structure. Interface analysis is vital to solving such problems.
X-ray Photoelectron Spectroscopy or XPS or ESCA:
- Quantitative elemental composition of surface contamination
- Surface chemistry with complete chemical phase identification
- Extremely high sensitivity with an analysis depth of 8nm and sensitivity for less than 0.1 monatomic layer
- Verification of surface cleanliness
- Surface chemistry changes caused by cleaning agents, both improvements and residues left
- Ion sputter gun removal of surface contamination to judge its thickness
- Quantitative elemental and chemical phase identification of interfacial contamination after adhesive failure, peel, or pull test
- Interphase chemistry of interfaces with adhesive bonding – adhesives often have different compositions near interfaces such as higher concentrations of silanes and amines
- Depth profile through thin layers at a surface for elemental analysis as a function of depth
- Analyze residues left after evaporating water or other solvents
- Identify plasticizers, fire retardants, slip agents, and cross-linking agents in polymers or plastics which often become more concentrated on a surface or at an interface between materials. These can cause delaminations and adhesive bond failures.
- Examine the concentration of an element or chemistry across an interface between two solid materials due to inter-diffusion due to heating, sputter deposition of the materials, or due to chemical reactions between the two materials
- Characterize metal alloy surface oxide layers whose elemental composition differs greatly from the bulk metal elemental concentrations such as the heightened concentration of chromium in stainless steel oxides. The surface oxide may have strong chemical gradients in it over very short distances.
- Determine the chemistry of fracture surfaces in interfacial zones of a material such as a ceramic or an intergranular failure of a metal alloy.
FTIR or Infrared Spectroscopy:
- Identification of organic functional groups and some inorganic functional groups in thicker contaminant layers using ATR or specular reflectance for greater surface sensitivity
- Bulk or near-surface organic material composition for comparison to surface organic composition
- Identify plasticizer or fire-retardant segregated to the surface of a plastic/polymer material
- With a sampling depth of 1000 to 2000 nm, FTIR can sometimes peer through an overlayer film of organic material into an organic substrate material, also detecting anything different from the two materials at the interface between them
- Often used to determine the nature of polymer degradation at a surface or an interface.
GC-MS or Gas Chromatography – Mass Spectroscopy:
- Plastic or elastomeric materials adhesively bonded to one another or other materials may have additives that migrate to surfaces and interfaces, where they may cause adhesive bonding failures in time. Measure and detect these additives.
Microscopic Examinations:
- SEM with digital imaging to reveal surface inclusions and surface deposits
- Inspection microscope, digital imaging for same
- Metallographic microscope, digital imaging, Nomarski phase contrast, for same
- White light interference microscopy or profilometry for surface deposits
- Optical microscopy can often look through a transparent overlayer material to see a deeper layer of material. Sometimes, defects in the lower material or degradation chemical reactions are then seen.
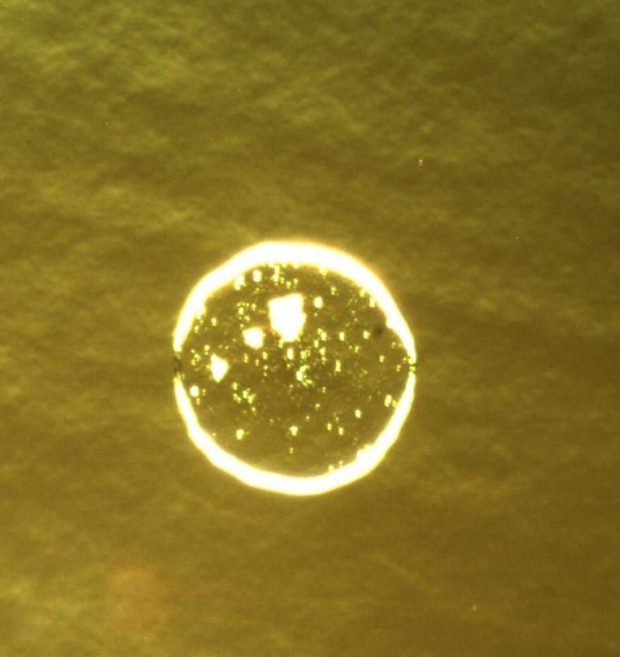
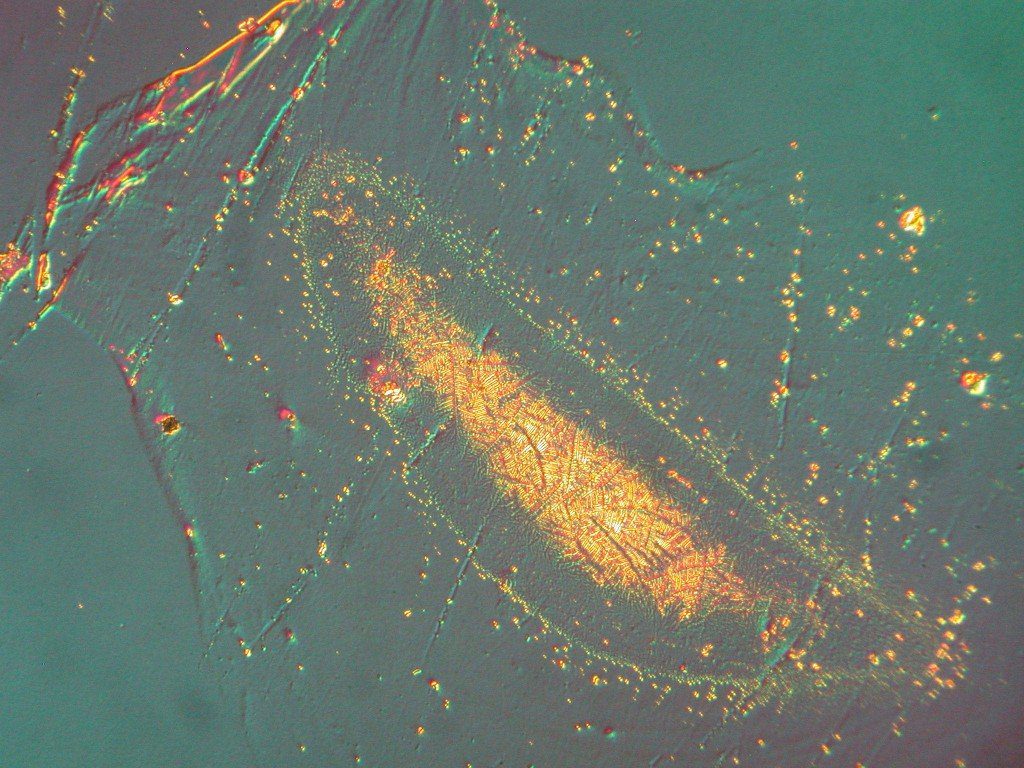
Effect of interfacial contamination on adhesion to the interface using pull tests.
Surface cleaning technique evaluations:
- CO2 snow jet cleaning, in-house capability, but learn more about CO2 snow jet cleaning at Applied Surface Technologies
- Solvent-based cleaning
- Microwave plasma cleaning
- Check cleaning effectiveness with above analytical capabilities
- Determine changes in surface chemistry of plastics caused by plasma cleaning
Electrochemistry or Corrosion Testing:
- Determine whether oxide film protects metal surface, which it may not due to contaminants, the attacking chemical agents, or a combination of both
- Determine breakdown potential of passive film on metal or pitting potential, which is affected by contamination and the corrosive media
- Determine the corrosion rate of a particular metal surface in a given electrolyte – changes in the surface oxide change the performance
- Measure the effectiveness of a coating or protective film in preventing corrosion as a function of time, applied potentials, and corrosive ions
Applications:
- Electronics contamination
- Packaging materials contamination
- Medical device contamination
- Composite materials fiber surface conditions and particulates surface chemistry or contamination affecting matrix bonding
- Adhesive bonding preparation and cause of bonding failures
- Corrosion due to contamination
- Analysis of intermetallic formation at an interface
- Analysis of the transition from surface microclusters of graphitic carbon to carbide formation in the bulk metal
- Analysis of the degree of depletion of magnesium near a surface in heated magnesium aluminum spinel
- Analysis of the depth of degradation of a material subjected to heat, UV radiation, or other radiation