Below is a detailed explanation of the corrosion analysis & electrochemistry work that we do.
Principles of Corrosion
Corrosion takes place when a metal surface reacts with its environment. There are certain essential components that are needed for a corrosion reaction to proceed; an anode, a cathode, an electrolyte with oxidizing species, and some direct electrical connection between the anode and cathode. A typical situation might involve a piece of metal that has anodic and cathodic regions on the same surface. If the surface becomes wet, corrosion may take place through ionic exchange in the surface water layer between the anode and cathode. Electron exchange will take place through the bulk metal. Corrosion will proceed at the anodic site according to a reaction such as
M —› Mn+ + ne– ,
where M is a metal atom, and n is the number of electrons lost during anodic oxidation. The resulting metal cations (Mn+) are available at the metal surface to become corrosion products such as oxides, hydroxides, etc. The liberated electrons travel through the bulk metal (or another low resistance electrical connection) to the cathode, where they are consumed by cathodic reactions such as
O2 + 2H2O + 4e– —› 4OH–
(example given for dissolved oxygen in neutral pH water).
Case history examples from our corrosion analysis & electrochemistry testing:
Evaluating Chemical Plant Intergranular Corrosion with Metallography and XPS Chemical Analysis
Electrochemical Qualification of Biomedical Implantable Devices
Exposure Testing for Materials Selection
Sensitization of 304 Stainless Steel Using the ASTM G108 Test Method
Graphitic Corrosion in Grey Cast Iron Investigation
Soil-Side Chloride-Induced Stress Corrosion Cracking Under Insulation Investigation
Capabilities of DC Electrochemistry for Corrosion Analysis
Potentiostatic, galvanostatic, and potentiodynamic polarization techniques allow for the controlled polarization of metal surfaces in electrolytes of interest, in order to directly observe cathodic and anodic behaviors, which are key in corrosion testing. Corrosion reactions can be monitored or driven at the surface of a desired metal sample, and a variety of characteristics related to the metal/environment pairing can be determined. Examples of characteristics of interest in corrosion analysis include open circuit or corrosion potential, instantaneous corrosion rate, passivation behavior, pitting potential and susceptibility, crevice corrosion susceptibility, sensitization of heat affected zones, and galvanic corrosion behavior of dissimilar metal pairs.
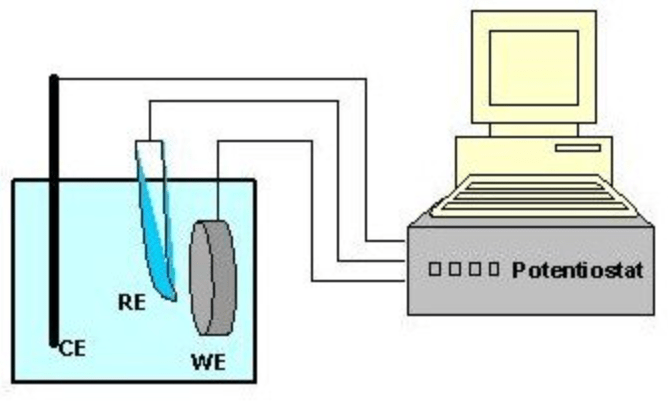
Polarization experiments in corrosion analysis are performed with a computer-controlled potentiostat. A constant or a varying DC potential (potentiostatic, potentiodynamic, respectively), or a constant DC current (galvanostatic) is applied to the metal of interest while it is immersed in the electrolyte. The metal sample is referred to as the working electrode (WE). The reference electrode (RE) is typically selected to be very stable under the selected test conditions, and is used to monitor and maintain potential at the WE surface. Ionic current is passed through the electrolyte between the counter electrode (CE) and the WE, and electron current is passed between the CE and the WE through a low resistance connection provided and monitored by the potentiostat. Evaluation of the current/potential behavior under a variety of conditions leads to the understanding of a range of corrosion phenomena.
Corrosion Analysis & Electrochemistry Applications
- Corrosion product identification and analysis
- Industrial and chemical plant corrosion and chemical attack
- Alloy selection appropriate to use and environment
- Estimate remaining lifetime of water pipes
- Equipment corrosion
- Crevice corrosion analysis
- Electronics corrosion
- Local corrosion analysis
- Aircraft corrosion
- Electroplating analysis
- Marine corrosion
- Pipeline corrosion
- Galvanic corrosion due to dissimilar metals, ion transfer, non-metallic conductors, and metal ion deposition (cementation on aluminum alloys)
- Bridge corrosion
- Biomedical implant corrosion
- Reinforced concrete corrosion
- Stainless steel sensitization
- Undercoat corrosion
- Weld corrosion and heat affected zone corrosion
- Intergranular corrosion analysis
- Stress corrosion cracking analysis
- Flux-induced corrosion analysis
- Undercutting and occluded cell corrosion analysis
- Pin-hole leaks in copper pipe and tubing
- Identify selective leaching and cause (i.e. dezincification of brass or graphitic corrosion of cast iron)
- Identify velocity affected corrosion or erosion
ASTM Test Methods for Corrosion Analysis
- ASTM G5 – Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements
- ASTM G59 – Standard Practice for Conducting Potentiodynamic Polarization Resistance Measurements
- ASTM G61 – Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys
- ASTM G108 – Standard Test Method for Electrochemical Reactivation (EPR) for Detecting Sensitization of AISI Type 304 and 304L Stainless Steels
- ASTM F746 – Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials
- ASTM F2129 – Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices
Please reach out to us about your electrochemistry and corrosion testing needs.