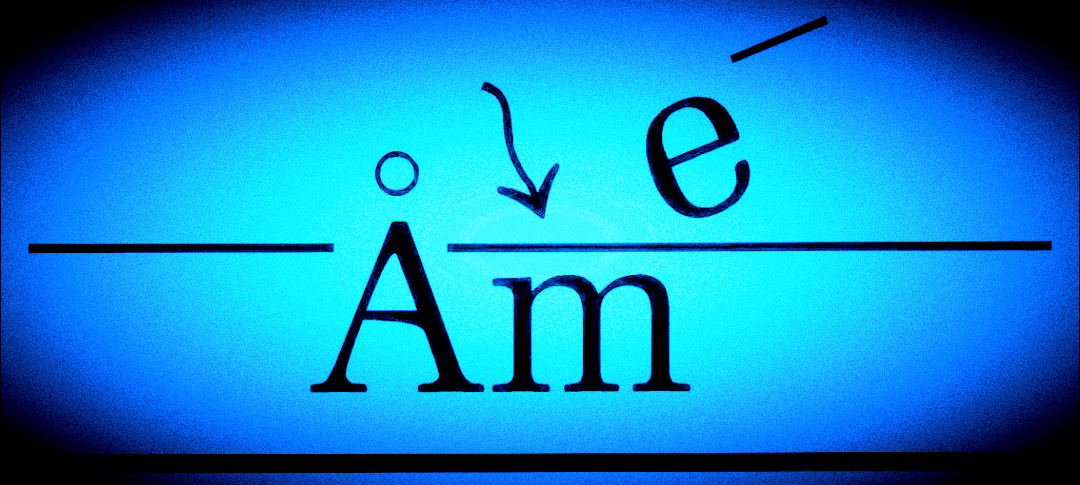Introduction
Gas Chromatography-Mass Spectroscopy (GC-MS) combines the GC and MS techniques to separate and identify organic compounds in mixtures of organic compounds. In GC the mobile phase is a carrier gas, usually an inert gas such as helium or nitrogen. The stationary phase is a microscopic layer of liquid or polymer absorbed on an inert solid support inside a capillary tube of glass or metal called a column. In GC a sample is vaporized when injected into the head of the column. The difference in the chemical properties between different molecules in a mixture and their relative affinity for the stationary phase of the column will promote separation of the molecules as the sample travels through the column. The molecules are absorbed on the column wall and then elute (come off) from the column at different times. Different molecules have different dwell times in the absorbed state on the column wall before desorbing and moving down the column. This process is repeated many times. Different column surface chemistries and different column lengths are used for optimal results depending upon what is being analyzed. This allows the mass spectrometer which is located downstream of the GC to break each molecule into ionized fragments and detect these fragments using their mass-to-charge ratios. In addition to mass spectroscopy, we use a Flame Ionization Detector (FID) and a Thermal Conductivity Detector (TCD). We can routinely detect and identify chemical species at parts per billion (ppb) concentrations from gas and liquid samples of a few microliters or solid samples of a gram or less. The schematic diagram of our GC-MS instrument is shown below in Figure 1.
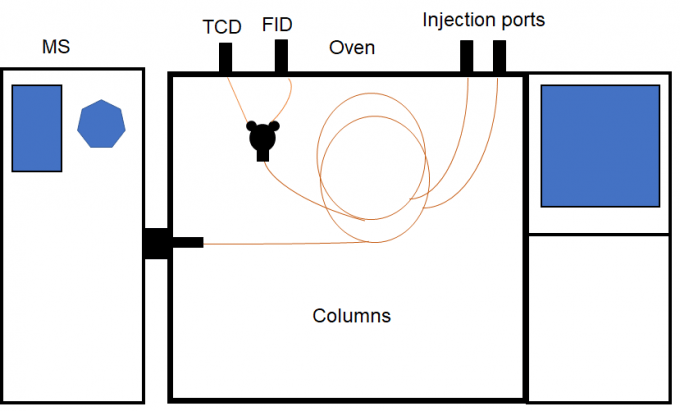
Our GC-MS system is a Thermo Fisher Trace 1310 ISQ 7000 with VPIU equipped with both FID and TCD detectors.
GC-MS Methods of Sample Extraction
Solvent extraction: Solvents such as hexane, dichloromethane, acetone and others may be used to extract chemicals so they can be injected into the GC.
HS-SPME: The volatile organic compounds present in a sample can be extracted using the headspace solid-phase micro-extraction (HS-SPME) method. 65 mm PDMS/DVB, stableflex fiber assembly is used to adsorb the volatiles. In a typical analysis, 3g of the sample is put into a 15 ml vial. The vial is sealed with a cap having a PTFE/silicone septa. The vial is conditioned at 50oC for 30 min prior to headspace SPME sampling. Then the SPME syringe is introduced through the septum and exposed to the sample in the headspace for 30 minutes, followed by desorption in the GC inlet at 250 oC.
Applications of GC-MS
GC-MS techniques can be utilized for the following applications
- Identifying and quantifying organic compounds in mixtures
- Detecting and measuring trace contaminants in liquids and gases
- Detecting and measuring Bisphenol A (BPA) in polymers / plastics
- Detecting and measuring Polybrominated Biphenyls and Polybrominated Diphenyl Ethers for RoHS
- Detecting and measuring the Phthalate organic compounds restricted by RoHS
- Detecting many organic compounds restricted by California Prop65
- Analyzing additive components of polymers extracted by solvent extraction
- Analyzing the volatile components of adhesives and sealants during their curing processes
- Analyzing the volatile organic compound (VOC) emissions from pesticides, paints, rugs, wall coverings, foam roofing insulation, as examples
- Identifying trace short chain length silicone impurities
- Detecting corrosive phenolic compounds in foam roofing insulation materials
- Identifying outgassed gases and vapors causing odors
- Identifying organic contaminants removed from surfaces
- Identifying residual solvents in products, including in pharmaceutical products
Example 1: Identification and separation of various small-molecule siloxanes (Silicones)Example 1: Identification and separation of various small-molecule siloxanes (Silicones)
We analyzed a mixture of cyclic and linear siloxanes by GC-MS. Eight different siloxanes were mixed together to test our ability to separate and identify them with our GC-MS and an appropriate column for the purpose. Given below are the GC and mass spectra for this mixture of small-molecule siloxanes. We were able to do an excellent job of both separating and identifying these siloxanes as an example of our extensive expertise in detecting silicone contaminants.
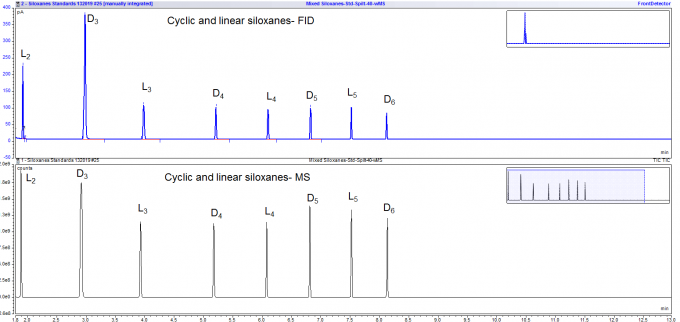
Figure 2. The upper spectrum shows the FID results separating the 8 linear and cyclic siloxanes and the lower spectrum is the MS separation of these siloxanes.
| Cyclic Siloxanes | Linear Siloxanes |
|---|---|
| D3: hexamethylcyclotrisiloxane | L2: hexamethyldisiloxane |
| D4: octamethylcyclotetrasiloxane | L3: octamethyltrisiloxane |
| D5: decamethylcyclopentasiloxane | L4: decamethyltetrasiloxane |
| D6: dodecamethylcyclohexasiloxane | L5: dodecamethylpentasiloxane |
Example 2: Sensitivity of GC-MS
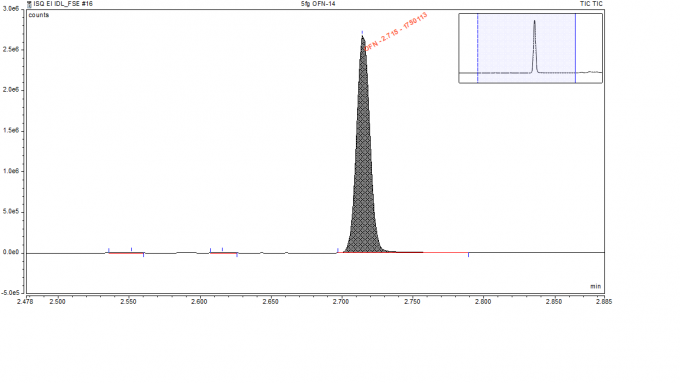
A standard 5 fg/µL Octafluoronaphthalene (OFN) in iso-octane solvent was used to evaluate the sensitivity of our mass-spectroscopy (MS) instrument. OFN is an inert, non-polar compound, ideal for the performance assessment of the sensitivity of a GC-MS. Our GC-MS system configured with the advanced electron ionization (AEI) source delivers a very high sensitivity as determined by the injection of 5 fg of OFN and its ready detection.
Example 3: Flame Ionization Detector Sensitivity
20 µg/ml each of three aliphatic straight chain alkanes, n-Dodecane (C12), n-Tetradecane (C14) and n-Hexadecane (C16), in hexane solvent were used to evaluate the sensitivity of our Flame Ionization Detector (FID).
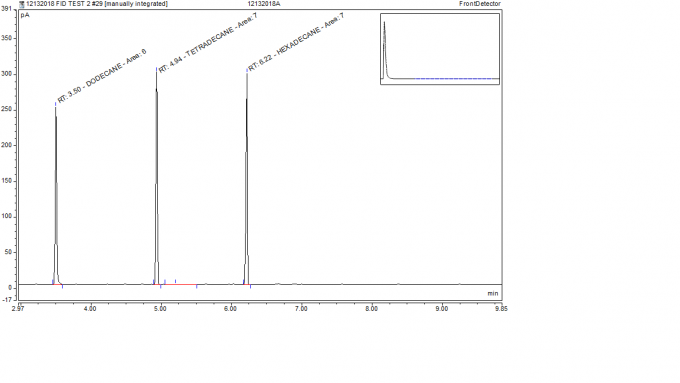
n-Hexadecane (C16) in hexane
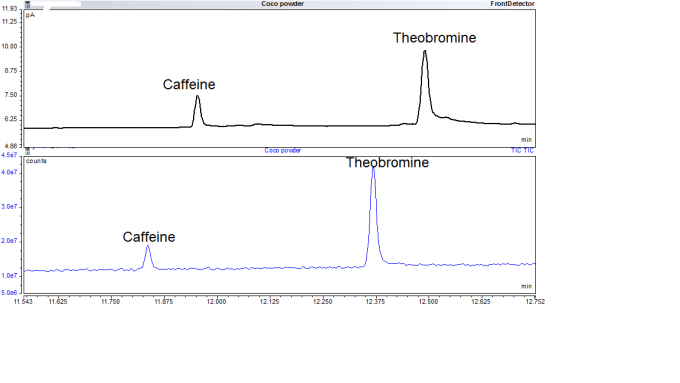
Example 4: The GC-MS Analysis of Caffeine and Theobromine
Caffeine and theobromine were extracted from coco powder and analyzed by GC-MS. The GC-MS chromatograph of caffeine and theobromine is shown in Figure 5. Before analysis, coco powder was treated in hot water for 30-45 mins. Caffeine and theobromine were then extracted from aqueous solution into dichloromethane using the solvent extraction method. During solvent extraction, caffeine and theobromine are more highly miscible in dichloromethane than water, which transfers them into dichloromethane. The dichloromethane was then dried over anhydrous sodium sulfate after separating it from the aqueous solution, before analysis by GC-MS. This analysis preceded a project to determine if a prior filling of a transportation vessel with liquid chocolate might have contaminated a later shipment of another liquid.
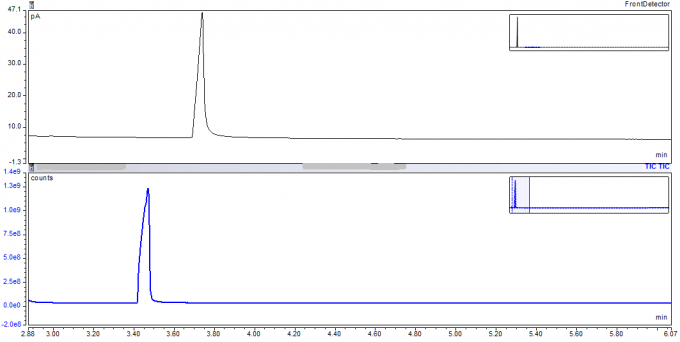
Example 5: A check of the column for contamination from prior analyses before looking for impurities in a propylene glycol customer sample
We used a known pure propylene glycol sample to test our column for any impurities from prior investigations so we could proceed with a customer project to check for impurities in propylene glycol taken from a tanker. Great care is needed to flush columns of chemicals run through them from prior analyses. The FID and the mass spectroscopy data below both proved that we had successfully flushed impurities out of our column.
Example 6: Analysis of honey samples using different sample preparation techniques to demonstrate the ability of GC-MS to analyze complex mixtures of organic compounds including the removal and identification of the volatile species.
Standard Test Methods
ASTM D1945 – Standard Test Method for Analysis of Natural Gas by Gas Chromatography
ASTM D1946 – Standard Practice for Analysis of Reformed Gas by Gas Chromatography
ASTM D3415 – Standard Practice for Identification of Waterborne Oils
ASTM D5830 – Standard Test Method for Solvents Analysis in Hazardous Waste Using Gas Chromatography
ASTM D6228 – Standard Test Method for Determination of Sulfur Compounds in Natural Gas and Gaseous Fuels by Gas Chromatography and Flame Photometric Detection
ASTM D6420 – Standard Test Method for Determination of Gaseous Organic Compounds by Direct Interface Gas Chromatography – Mass Spectrometry
ASTM D6886 – Standard Test Method for Determination of the Weight Percent Individual Volatile Organic Compounds in Waterborne Air-Dry Coatings by Gas Chromatography
ASTM D7833 – Standard Test Method for Determination of Hydrocarbons and Non-Hydrocarbon Gases in Gaseous Mixtures by Gas Chromatography
ASTM D8143 – Standard Test Method for Determination of the EU-8 List of PAH Compounds in Carbon Black
EPA Method 8015D – Analysis of Nonhalogenated Organics Using GC/FID
Please contact us regarding your materials analysis needs. We will be more than happy to discuss your concerns and how we may be able to help you.