Illustrative Example: Analysis of a Feldspar Mineral
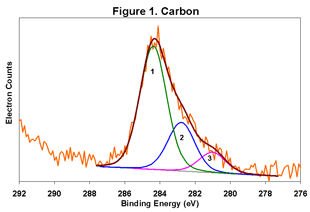
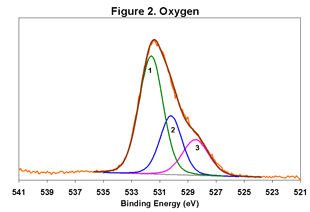
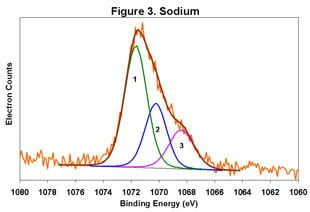
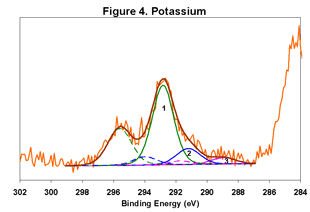
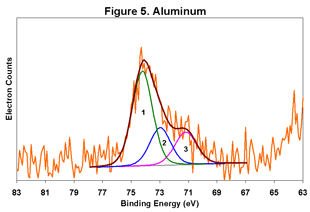
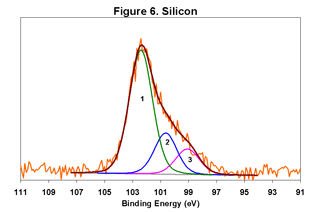
Figures 1-6 below display the high-resolution XPS spectra for elements on the surface of a freshly ground feldspar sample. We use high-resolution analyses to determine the various chemical species present in the sample.
Analysis Details
In each spectrum, we identified three component peaks corresponding to three different chemical phases:
- Phase 1: Na₂O • 0.76 K₂O • 1.59 Al₂O₃ • 12.64 SiO₂ • 0.84 H₂O
- Phase 2: Na₂O • 0.33 K₂O • 1.26 Al₂O₃ • 7.86 SiO₂ • 8.48 H₂O
- Phase 3: Na₂O • 0.19 K₂O • 1.62 Al₂O₃ • 7.25 SiO₂ • 9.79 H₂O
Feldspar Varieties
Feldspar comes in various types, including Soda Feldspar (Na₂O • Al₂O₃ • 6 SiO₂), Potash Feldspar (K₂O • Al₂O₃ • 6 SiO₂), and Calcium Feldspar (CaO • Al₂O₃ • 2 SiO₂). The feldspar we analyzed is a complex compound with three distinct chemical phases. Phase 1 is the major component, while Phases 2 and 3 are minor. By taking advantage of the differential charging of these species, we could identify each phase separately. Without this differential charging, the material would show a weighted average of these three compositions.
Key Findings
The main phase has a relatively low degree of hydration, whereas the two minor phases are highly hydrated. In certain applications, the presence of these highly hydrated phases is significant.
XPS High-Resolution Spectra
For a detailed view of the spectra, refer to Figures 1-6. These figures illustrate the high-resolution XPS analysis of the feldspar sample.
Additional Information
To learn more about XPS high-energy resolution chemical analysis, visit our XPS page.
Contact Us
If you need further assistance with XPS analysis or other material evaluations, please contact us today. Our experts are ready to help with accurate and detailed assessments.