Corrosion Case Study: Electrochemical Qualification of Biomedical Implantable Devices
Implantable biomedical devices must be qualified by a series of tests prior to approval for use by the FDA. Corrosion behavior of the raw material and the finished devices can be tested using ASTM F746, “Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials,” and ASTM F2129, “Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices,” respectively. Both of these tests are available at AME. Both are destructive tests, meaning that the material is expected to undergo forced corrosion during the procedure. The example below shows an application of ASTM F2129.
In this corrosion case study, nickel-titanium alloy stents (implantable biomedical devices) were subjected to electrochemical testing according to ASTM F2129. Each stent was received in testing condition and was mounted at the AME laboratory. The testing was performed in commercially obtained Hanks’ solution with a starting pH of approximately 7.1. Fresh solution was used for each stent. Nitrogen gas was bubbled through the solution to remove excess dissolved oxygen. The solution was heated to an average temperature of 37°C for the duration of the measurement.
Each device is exposed to the deoxygenated and heated solution for one hour at open circuit potential prior to commencing the cyclic polarization scan. Scans begin at 100 mV negative to the open circuit potential, then proceed in a forward direction at 10 mV per minute through the cathodic/anodic transition and continue on to a potential beyond the onset of pitting. At a preset current the scan direction is reversed and proceeds in the negative direction until a protection potential is found or the preset final current is achieved.
The resulting potentiodynamic scans are shown in Figure 1. The data format shows the applied potential on the vertical axis and the logarithm of the resulting current density, measured in mA/cm2, on the horizontal axis. The open circuit corrosion potential (Ecorr, where the cathodic/anodic transition takes place), the breakdown potential that indicates the onset of pitting (Eb, also sometimes called Epit), and the protection potential below which pits will no longer propagate (Ep, also sometimes called Eprot), are all labeled in Figure 1. The hysteresis loop formed by each polarization scan is an indication of the susceptibility of this material to pitting attack. The best performing materials in terms of pitting attack have relatively little or no hysteresis effect.
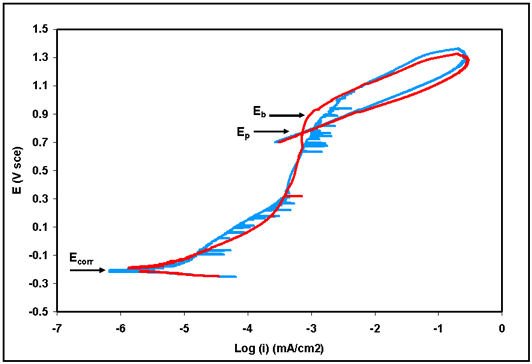
The results required by ASTM F2129 for each cyclic polarization scan are given in Table 1. All potentials are given versus the saturated calomel (SCE) reference electrode. Ecorr and icorr (the current density at Ecorr, used to determine corrosion rate), as well as the breakdown or pitting potential (Eb), and the protection potential (Ep) are determined directly from the scans. Ep is reported if the reverse scan intersects with the forward scan, thereby closing the hysteresis loop. Final potential Ef is reported if the hysteresis loop is not closed by the end of the scan, indicating no recovery and severe susceptibility to pitting. In the present example both stents recovered, meaning that existing active pits were “shut off” at potentials less than Ep. The pH was also measured before and after each polarization in keeping with the requirements of the standard. These results showed good repeatability and an acceptable pitting behavior for the material in question.
Table 1. Results of the ASTM F2129 Cyclic Potentiodynamic Scans
| Test Specimen | Ecorr (mV) |
icorr (mA/cm2) |
Eb (mV) |
Ep (mV) |
Ef (mV) |
pH Start |
pH End |
|---|---|---|---|---|---|---|---|
| Stent 1 | -208 | 5.4 x 10-6 | 840 | 792 | N/A | 7.1 | 8.0 |
| Stent 2 | -201 | 3.3 x 10-6 | 837 | 754 | N/A | 7.2 | 8.0 |
Please contact us regarding your electrochemistry and corrosion testing needs.