Illustrative Example: Analysis of an Emerald Gemstone
Overview
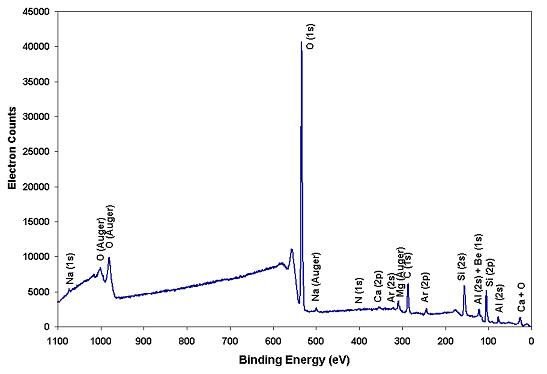
Figure 1 below presents the XPS elemental survey spectrum of an emerald gemstone surface. To excite photoelectrons from the surface, we used a small-spot Al x-ray source. The XPS survey spectrum includes photoelectrons, Auger electrons, and secondary electrons, all of which relate to the binding energies of the electrons in photoelectron peaks. These binding energies help us identify the elements from which the electrons were emitted.
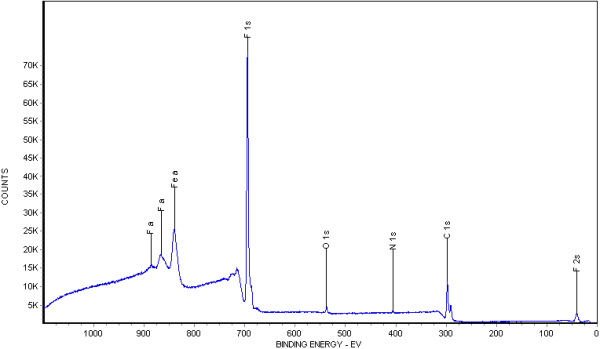
Before we collected the survey spectrum, we sputtered the emerald surface with 4 keV Ar ions for 12 seconds to remove surface-adsorbed hydrocarbon impurities. We then conducted the survey analysis within a common binding energy range of 0-1100 eV, using a step size of 0.5 eV. This finer step size improves the accuracy of peak area measurements for more precise quantitative results. Figure 2 provides an enlarged view of the 0-250 eV binding energy region from Figure 1, highlighting the high signal-to-noise ratio that enhances both quantitative accuracy and detection sensitivity.
Figures
- Figure 1: XPS elemental survey spectrum of the emerald gemstone surface
- Figure 2: Enlarged XPS survey spectrum of the 0-250 eV binding energy region
Elemental Composition
The elemental compositions (at.%) on the surface of the emerald detected by XPS are listed in Table 1 below.
| Table 1 | |
|---|---|
| Element | At.% |
| Na | 0.79 |
| Al | 4.78 |
| Mg | 0.91 |
| Be | 6.12 |
| Si | 16.90 |
| C | 14.51 |
| Ca | 0.49 |
| N | 0.55 |
| O | 54.97 |
A typical emerald is a beryl or beryllium aluminum silicate with the formula Be₃Al₂Si₆O₁₈ (3BeO • Al₂O₃ • 6SiO₂). In some cases, Na, Li, and Cs may replace Be, reducing the BeO content. Additionally, emeralds may contain Mg, Ca, Cr, and other elements. The carbon detected is due to surface-adsorbed hydrocarbons and graphitic carbon inclusions.
Comparison with Typical Beryl
Table 2 compares the at.% of components between the emerald from this study and a typical beryl, after removing the carbon.
| Table 2 | |||
|---|---|---|---|
| Component | Present Study (At.%) | Beryl (at.%) | % Beryl Value |
| BeO | 14.32 | 20.69 | 69.21 |
| Al2O3 | 13.98 | 17.24 | 81.11 |
| SiO2 | 59.31 | 62.07 | 95.55 |
| Na2O | 1.38 | – | – |
| MgO | 1.06 | – | – |
| CaO | 0.57 | – | – |
| Others | 9.38 | – | – |
The additional elements found in this gem have reduced the BeO and Al₂O₃ content compared to a typical beryl. These substitutions may account for some of the flaws observed microscopically in this gemstone and its lack of brilliance after removing covering oils.
Additional Information
For a related example, see our report on the surface analysis of coated and stripped polyethylene fibers.
We also discuss whether quantitative elemental composition analysis is best obtained using EDX, XPS, or both techniques elsewhere.
Contact Us
For more information or assistance with XPS analysis, please contact us. Our experts are ready to provide detailed evaluations and support.