How the surface chemistry of an aged silicon oxynitride film is determined by XPS analysis of the surface elemental composition:
The elemental spectrum of the surface was:
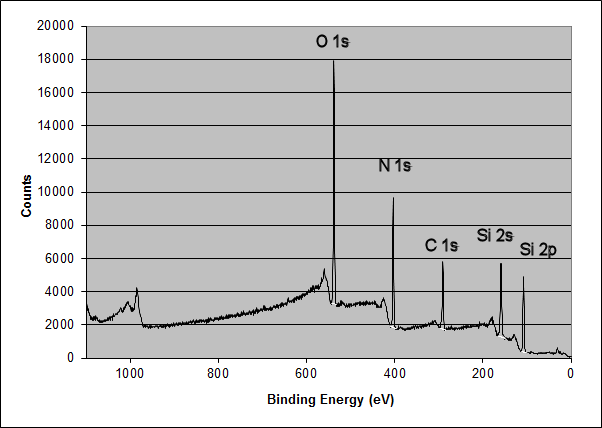
Aged Silicon Oxynitride Film Surface Chemistry:
The quantitative analysis says we have: Si 28.003 O29.015 N 20.685
Normalizing this to the silicon concentration, we have: Si O1.036 N 0.739
Now we note that if oxygen is all in the oxide form, there would be an excess of negative charge, since oxygen as oxide is -2 valance and N as nitride is -3 valance. Then 2 (-1.036) + 3 (-0.739) = -4.289, which is out of balance with the +4.000 charge on the silicon. From this we conclude that some of the oxygen is in the form of hydroxyl groups on the surface of the film.
The chemistry must then take the form: Si Ox N0.739 (OH)z, where
x + z = 1.036 from the total oxygen concentration and
2x + z = 4 – 3 (0.739) = 1.783 from the charge requirement given the known nitride concentration.
Solving for x and z we find that the surface chemistry of the aged silicon oxynitride film is:
Si O 0.747 N 0.739 (OH) 0.289
Please contact us today with your surface chemistry concerns!