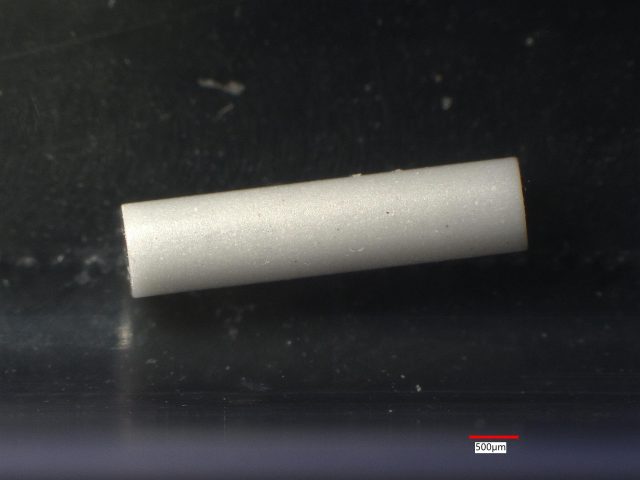
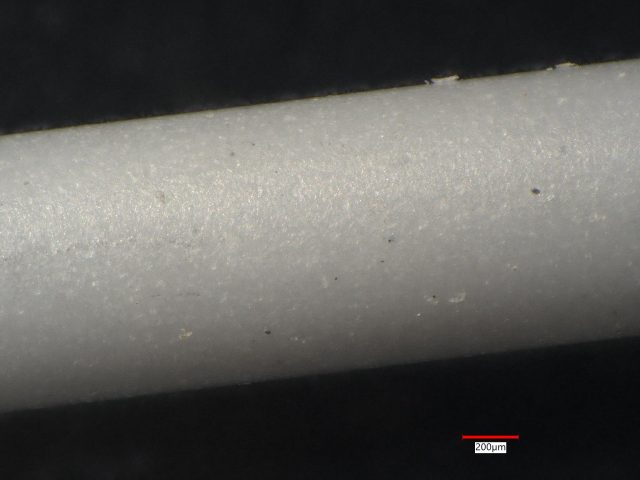
At Anderson Materials Evaluation, we specialize in advanced materials analysis for biomedical device applications. In this case study, we examine three small silicon nitride ceramic rods. Initially, we noticed appearance differences between the rods. However, our detailed analysis revealed that these rods have different compositions and are not pure silicon nitride.
Optical Microscopy Examination and LIBS Analysis
To conduct a thorough analysis, we used the Keyence VHX-7000N Digital Optical Microscope, which is paired with an EA-300 Laser-Induced Breakdown Spectroscopy (LIBS) unit. This advanced equipment allowed us to capture detailed images and analyze elemental compositions.
Optical Microscopy
Firstly, the digital microscope provides zoom magnification from 20 to 2000 times, using a 4K CMOS image sensor. The motorized sample stage and lens allow us to capture images from various distances above the sample and reconstruct images from the in-focus areas. As a result, this gives us a detailed view of features at higher magnification, similar to a Scanning Electron Microscope (SEM).
- Imaging Large Areas: The microscope can image extensive areas by automatically stitching multiple adjacent images together. This feature ensures a comprehensive view of the specimen.
- Lighting Options: The device provides axial through-the-lens and ring lighting and can illuminate the sample from various angles.
- Measurements and Analysis: We can perform both 2-dimensional and 3-dimensional distance measurements, particle size analyses, and grain-size analyses. Additionally, the instrument allows us to acquire surface topography profiles and surface roughness measurements through advanced automatic image analysis.
LIBS Analysis
The EA-300 LIBS elemental analyzer employs a 355 nm laser beam to focus on an area about 10 micrometers in diameter. This laser pulse ionizes material from the surface to a depth of approximately 5 to 7 micrometers. As the resulting plasma de-excites, it emits characteristic radiation across the deep ultraviolet, visible, and near-infrared spectrum (185 – 960 nm wavelength). We analyze the emitted light through the lens using a 3.19-megapixel CMOS image sensor, which identifies elements semi-quantitatively based on intensity and color data.
This tool enables us to analyze small areas and tiny contamination particles for elements including H, Li, Be, B, C, N, O, F, Na, Mg, Al, Si, P, S, and elements heavier than U.
Analysis Results
Sample 1: White Ceramic Rod
Appearance and Analysis:
Sample 1 is a white ceramic rod. Our LIBS analysis detected Si at 26 out of 27 sites. At one site, we also detected K and Na.
- Conclusion: This sample predominantly consists of silicon, with minimal contamination from potassium and sodium. It lacks significant impurities, presenting as almost pure silicon nitride.
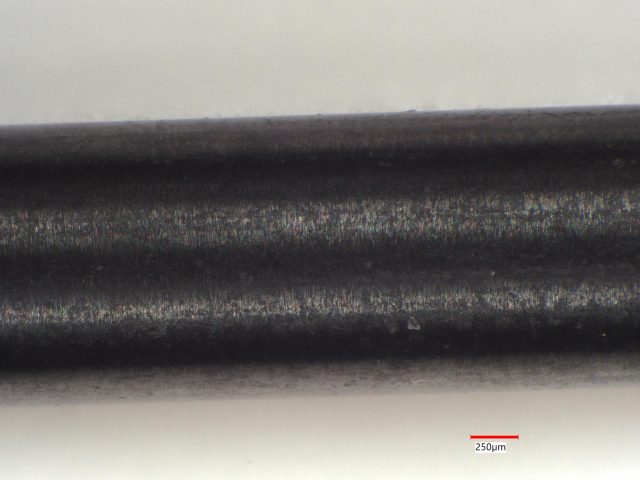
Sample 2: Gray Ceramic Rod
Appearance and Analysis:
Sample 2 exhibits a gray appearance, featuring black and near-white domains visible in the photomicrographs. This distinct appearance prompted further analysis.
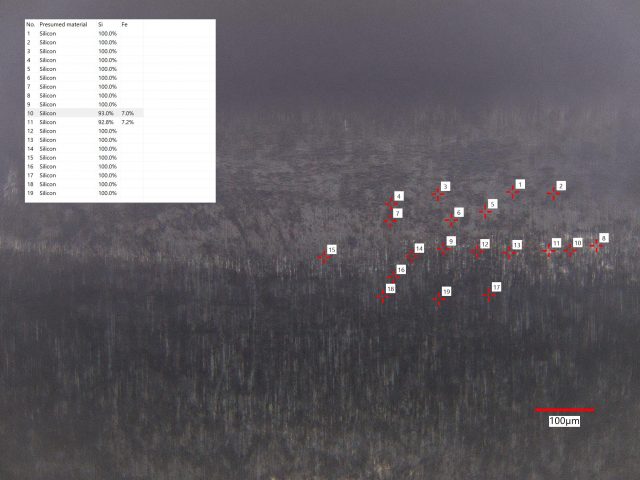
- LIBS Analysis: We conducted LIBS analysis on 30 sites, detecting Si at all sites, Ti at 12 sites, and Fe at one site. The Ti concentration ranged from 2.4 to 4.8 atomic percent (at.%), while the lone Fe concentration was 5.5 at.%.
- Conclusion: This sample consists of silicon but also contains titanium and iron impurities, contributing to its unique coloration and composition.
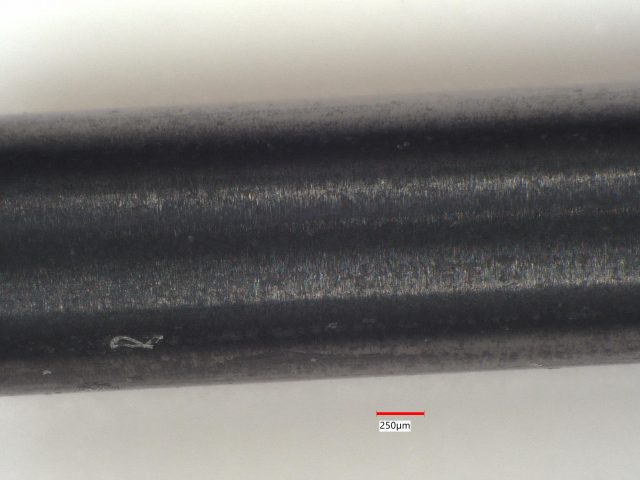
Sample 3: Another Gray Ceramic Rod
Appearance and Analysis:
Sample 3, as shown in the photomicrographs, features black and white local domains, similar to Sample 2.
- LIBS Analysis: We analyzed clusters of sites along the rod’s length, detecting Si at all 49 sites, Fe at two, and Al at two.
- Conclusion: This sample contains primarily silicon with some iron and aluminum impurities. These impurities influence its coloration and composition, making it distinct from a pure silicon nitride ceramic.
Optical Microscopy and LIBS Conclusions
Our comprehensive analysis of the three samples revealed the following:
- Sample 1: Predominantly white with minimal black spots, with LIBS detecting mostly silicon and trace amounts of potassium and sodium.
- Sample 2: Gray with black and white domains, exhibiting silicon as well as titanium and iron impurities.
- Sample 3: Gray with black and lighter gray domains, containing primarily silicon along with minor iron and aluminum impurities.
XPS Surface Analysis
To understand the surface chemistry, we performed X-ray Photoelectron Spectroscopy (XPS) analysis. This analysis focuses on the outermost layers of the material, providing surface composition information.
XPS System and Procedure
Our XPS system consists of a turbomolecular pumped introduction chamber, an ion-pumped sample preparation chamber, and an analysis chamber equipped with both ion and turbomolecular pumps. This system ensures minimal exposure to contaminants and accurate results.
- Pressure Control: Upon introducing samples into the Analysis Chamber, the pressure stabilizes in the low 10-8 Torr range, reaching the low 10-9 Torr range before data acquisition. This control minimizes cross-contamination.
Quantitative Elemental Concentration
Our XPS analysis provided quantitative elemental concentration results for each ceramic rod:
- Sample 1: The ratio of nitrogen to silicon (N/Si) was 0.8058, with a fraction (f) of 0.6044 compared to the 1.333 ratio of N to Si for pure silicon nitride.
- Sample 2: The N/Si ratio was 0.5761, with a fraction (f) of 0.4321, indicating a significant deviation from pure silicon nitride.
- Sample 3: The N/Si ratio was 0.5524, with a fraction (f) of 0.4143, further deviating from the pure silicon nitride standard.
These results underscore the presence of other elements and compounds beyond silicon nitride in each sample.
Surface Chemistry Conclusions
Our high-resolution XPS analysis unveiled key insights into the chemical composition of the ceramic surfaces:
Sample 1
- Composition: Contains both Si3N4 and Si3N2O3, with Si in the oxynitride form at a ratio of 0.80/2.87 = 0.279 compared to the nitride. Additionally, SiC is present, with Si in this form at a ratio of 0.055. SiO2 is also present at 0.551 times the amount of silicon nitride.
- Conclusion: This sample is composed of multiple forms of silicon, with almost as much Si in non-nitride forms as in silicon nitride.
Sample 2
- Composition: Includes Si3N4 and two distinct forms of silicon oxynitride (Si4N4O2 and Si4N2O5). The Si ratio compared to silicon nitride is 1.253 and 0.505, respectively. Si in silica form is present at a ratio of 3.17 compared to silicon nitride.
- Conclusion: This sample contains more silicon oxynitrides and silica than silicon nitride, resulting in a complex composition.
Sample 3
- Composition: Comprises both Si3N4 and Si3N2O3, with a similar ratio of Si in the oxynitride form to silicon nitride as in Sample 1 (0.68/2.38 = 0.286). SiO2 is present at 1.353 times the amount of silicon nitride.
- Conclusion: This sample has more silica than silicon nitride, though not as much as the second sample. The ratio of non-nitride Si to silicon nitride is 1.639, indicating significant compositional complexity.
Additional Observations
The XPS analysis did not detect the small amounts of Ti found in Sample 2 by LIBS or the low Fe concentration found in Sample 3 by LIBS. This discrepancy suggests that Ti may be too deep for XPS detection in the second sample, while Fe concentration in the third sample might be too low or not present on the surface. However, XPS did detect Al and Ca on all ceramic surfaces, confirming that true silicon nitride constitutes less than half of the ceramic material at these surfaces. Much of the ceramic consists of silica and silicon oxynitride.
Contact Us
For more information or to discuss your material analysis needs, please contact our team. We are here to assist with inquiries and provide tailored solutions for your engineering and research requirements.