This document explains how we assess the purity of a calcium carbonate sample using two analytical methods available in our laboratories: thermogravimetric analysis (TGA) and wavelength-dispersive X-ray fluorescence (WD-XRF). These methods complement each other, giving us a fuller picture of the sample’s composition than either could on its own. This document will discuss how both analyses provide a comprehensive understanding of the sample.
Thermogravimetric Analysis (TGA) for Purity
Thermogravimetric analysis measures how a material’s weight changes as it heats up. We used TGA to determine how pure the calcium carbonate sample was by measuring how it broke down when heated. We heated the calcium carbonate sample in a nitrogen atmosphere from room temperature to 900°C using our ISI TGA-1000 instrument. The sample’s weight loss told us a lot about its purity.
The sample started to lose weight at around 615°C and continued until about 777°C. This is because calcium carbonate breaks down into calcium oxide (CaO) and carbon dioxide (CO2) at these temperatures. For a pure calcium carbonate sample, we expect a weight loss of 43.971% due to the release of CO2.
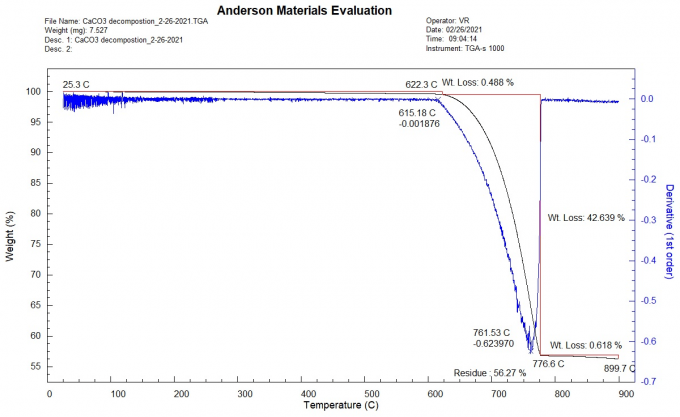
Figure 1 shows the TGA thermogram for the thermal decomposition of our calcium carbonate sample. The blue curve shows the first derivative of the sample weight curve with respect to temperature, indicating how quickly the sample lost weight.
- From room temperature to 615°C, the sample lost 0.488% of its weight.
- Between 615°C and 776.6°C, the weight loss was 42.639%.
- The maximum rate of weight loss occurred at 761.53°C.
- From 776.6°C to 899.7°C, the sample lost an additional 0.618%.
Overall, the sample showed a weight loss of 42.639%, which suggests that the sample was about 96.97% pure. However, impurities that decompose in the same temperature range as calcium carbonate could mean that the actual purity is somewhat lower.
Conclusions
Our TGA analysis indicates that the calcium carbonate sample has a purity of up to 96.97% by weight. The XRF analysis, however, suggests the purity cannot be greater than 96.38 wt.%. The XRF does not tell us how much of the 96.38 wt.% of the calcium is in the form of calcium carbonate. It might exist as CaO or CaCl2, for example.
The TGA result suggests that almost all of the calcium is in the form of calcium carbonate. Given that the primary impurities identified by XRF are sodium, magnesium, and strontium, magnesium carbonate is likely the main impurity reducing the calcium carbonate from its upper limit of 96.97 wt.%.
The maximum weight percent of magnesium carbonate is 0.493 wt.%, so the calcium carbonate content is almost certainly between 96.48 and 96.97 wt.%.
Contact Us for Your Testing Needs
If you need reliable and precise testing for your materials, we are here to help. Our laboratory is equipped with state-of-the-art instruments, and our experienced team is ready to assist you with your specific testing requirements. Whether it’s TGA purity analysis, thermogravimetric purity analysis, or XRF analysis of calcium carbonate, we have the expertise and equipment to deliver accurate results.
Please contact us for more information or to schedule a consultation. Our team is dedicated to providing top-notch service and assisting you with any questions or concerns regarding your materials testing needs.