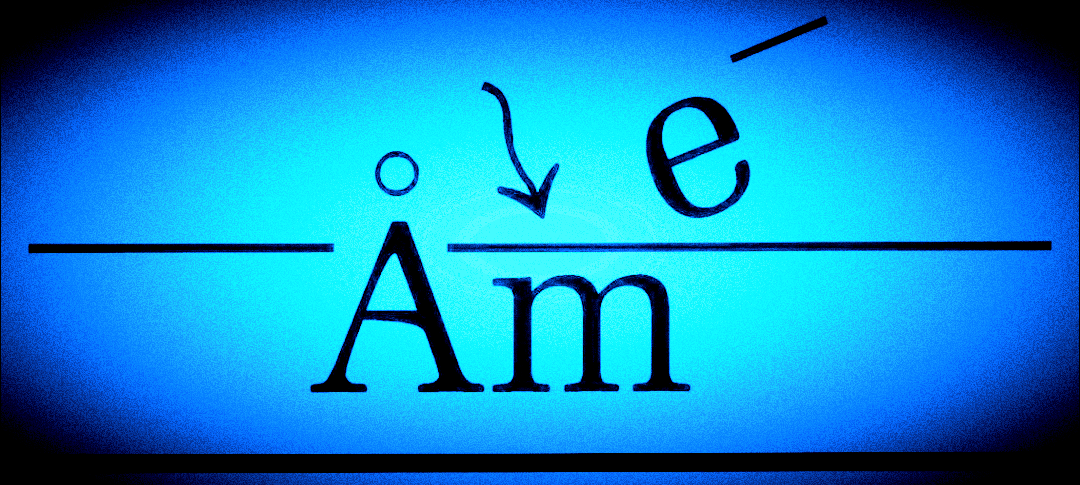Below is a detailed explanation of the corrosion analysis work that we do.
Principles of Corrosion
Corrosion takes place when a metal surface reacts with its environment. There are certain essential components that are needed for a corrosion reaction to proceed; an anode, a cathode, an electrolyte with oxidizing species, and some direct electrical connection between the anode and cathode. A typical situation might involve a piece of metal that has anodic and cathodic regions on the same surface. If the surface becomes wet, corrosion may take place through ionic exchange in the surface water layer between the anode and cathode. Electron exchange will take place through the bulk metal. Corrosion will proceed at the anodic site according to a reaction such as
M —› Mn+ + ne– ,
where M is a metal atom, and n is the number of electrons lost during anodic oxidation. The resulting metal cations (Mn+) are available at the metal surface to become corrosion products such as oxides, hydroxides, etc. The liberated electrons travel through the bulk metal (or another low resistance electrical connection) to the cathode, where they are consumed by cathodic reactions such as
O2 + 2H2O + 4e– —› 4OH–
(example given for dissolved oxygen in neutral pH water).
Case history examples from our corrosion analysis:
Graphitic Corrosion in Grey Cast Iron Investigation
Soil-Side Chloride-Induced Stress Corrosion Cracking Under Insulation Investigation
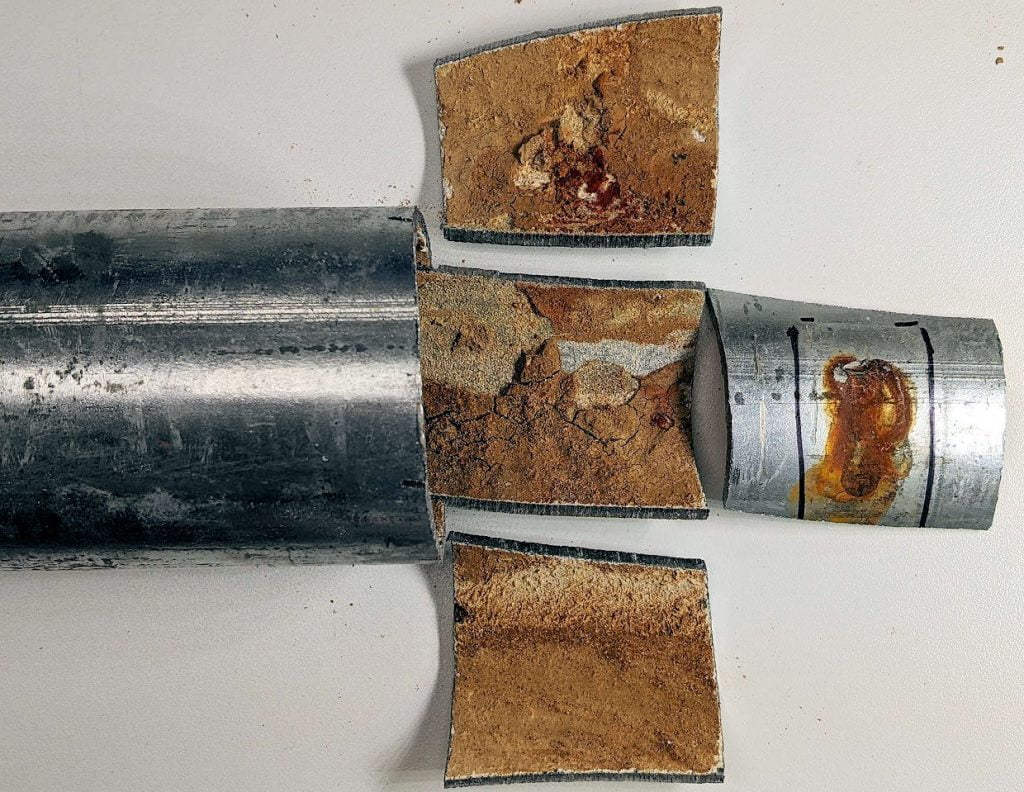
Microbiologically Influenced Corrosion (MIC) of Indoor Sprinkler System Galvanized Steel Pipe
Corrosion Analysis Applications
- Corrosion product identification and analysis
- Industrial and chemical plant corrosion and chemical attack
- Alloy selection appropriate to use and environment
- Estimate remaining lifetime of water pipes
- Equipment corrosion
- Crevice corrosion analysis
- Electronics corrosion
- Local corrosion analysis
- Aircraft corrosion
- Electroplating analysis
- Marine corrosion
- Pipeline corrosion
- Bridge corrosion
- Reinforced concrete corrosion
- Undercoat corrosion
- Weld corrosion and heat affected zone corrosion
- Stress corrosion cracking analysis
- Microbiologically influenced corrosion (MIC)
- Flux-induced corrosion analysis
- Undercutting and occluded cell corrosion analysis
- Pin-hole leaks in copper pipe and tubing
Please reach out to us about your corrosion testing needs.